Pfizer and BioNTech could request FDA authorization for children between 6 months and 5 years of age as early as today.
Members of the last age group to be authorized for COVID-19 vaccinations may soon get their due, and earlier than anticipated. Pfizer and BioNTech are expected to request emergency use authorization for their mRNA vaccine from the Food and Drug Administration as early as today, which would permit children between 6 months and 5 years of age to be vaccinated in a two-dose regimen.
The companies announced last fall that preliminary analyses of clinical trials of two vaccine doses in young children produced a protective effect in kids younger than 2 years old. But those results also suggested the response in kids 2 to 5 years old was not strong enough. As a result,they would evaluate how giving a third dose to kids aged 2 to 5 would impact their immune response.
If Pfizer and BioNTech waited for the results of the three-dose trials—which will likely end in March— regulatory approval would be unlikely to happen until late spring. The motivation is that, by filing for emergency use authorization now, children would be able to get their initial two doses without delay. Then kids aged 2 to 5 will be ready for a third shot by the time the additional clinical trials are done, if the data support use of that extra dose.
“We know that two doses isn’t enough, and we get that,” an unnamed source familiar with the situation told. “The idea is, let’s go ahead and start the review of two doses. If the data holds up in the submission, you could start kids on their primary baseline months earlier than if you don’t do anything until the third-dose data comes in.”Parents have been anxiously awaiting the go-ahead to vaccinate their small children.
México Últimas Noticias, México Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
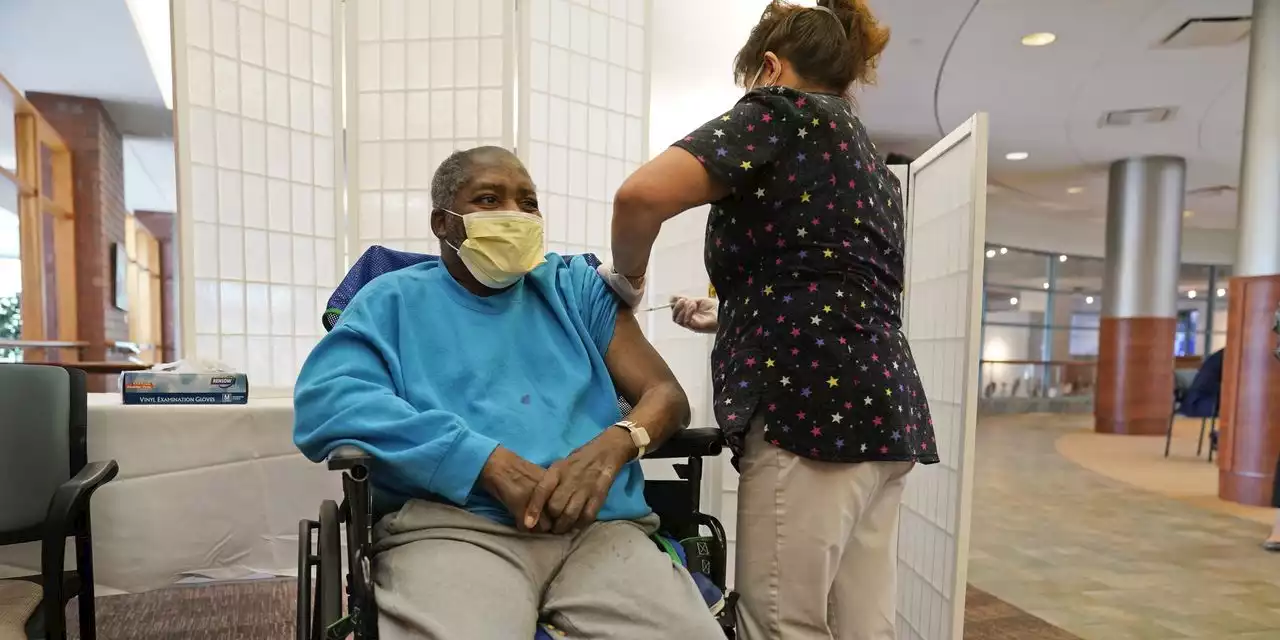 FDA Fully Approves Moderna’s Covid-19 Vaccine; U.S. Infections FallThe agency’s decision to make the company’s Spikevax shot the second fully approved Covid-19 vaccine came as case counts and hospitalizations in the U.S. continued to decline.
FDA Fully Approves Moderna’s Covid-19 Vaccine; U.S. Infections FallThe agency’s decision to make the company’s Spikevax shot the second fully approved Covid-19 vaccine came as case counts and hospitalizations in the U.S. continued to decline.
Leer más »
 Moderna's COVID-19 vaccine gets full FDA approvalModerna's COVID-19 vaccine called “Spikevax” has been given full approval from the U.S. Food and Drug Administration.
Moderna's COVID-19 vaccine gets full FDA approvalModerna's COVID-19 vaccine called “Spikevax” has been given full approval from the U.S. Food and Drug Administration.
Leer más »
 Moderna COVID vaccine gets full FDA approvalThe shot has been given to tens of millions of Americans since its emergency authorization over a year ago.
Moderna COVID vaccine gets full FDA approvalThe shot has been given to tens of millions of Americans since its emergency authorization over a year ago.
Leer más »
 Moderna COVID-19 vaccine gets full FDA approvalThe Moderna Covid-19 vaccine received full approval from the U.S. Food and Drug administration Monday, 11 months after the shot was given an emergency use authorization.
Moderna COVID-19 vaccine gets full FDA approvalThe Moderna Covid-19 vaccine received full approval from the U.S. Food and Drug administration Monday, 11 months after the shot was given an emergency use authorization.
Leer más »
 Moderna's COVID-19 vaccine granted full FDA approval, company saysModerna says the Food and Drug Administration has issued full approval for its COVID-19 vaccine for use in people aged 18 and older.
Moderna's COVID-19 vaccine granted full FDA approval, company saysModerna says the Food and Drug Administration has issued full approval for its COVID-19 vaccine for use in people aged 18 and older.
Leer más »
