The CDC's vaccine advisers have recommended COVID-19 vaccines for children under 5.
The panel unanimously agreed on Saturday that the vaccines made by Moderna and Pfizer should be open to young children.
Once she's signed off on it, the Biden administration said shots would be available as early as next week. On Wednesday, an FDA advisory panel unanimously found that both vaccines were “safe and effective” for use in children in that young age group.
México Últimas Noticias, México Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 COVID-19 vaccine: FDA authorizes 1st shots for kids under 5; CDC review nextBREAKING: U.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
COVID-19 vaccine: FDA authorizes 1st shots for kids under 5; CDC review nextBREAKING: U.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
Leer más »
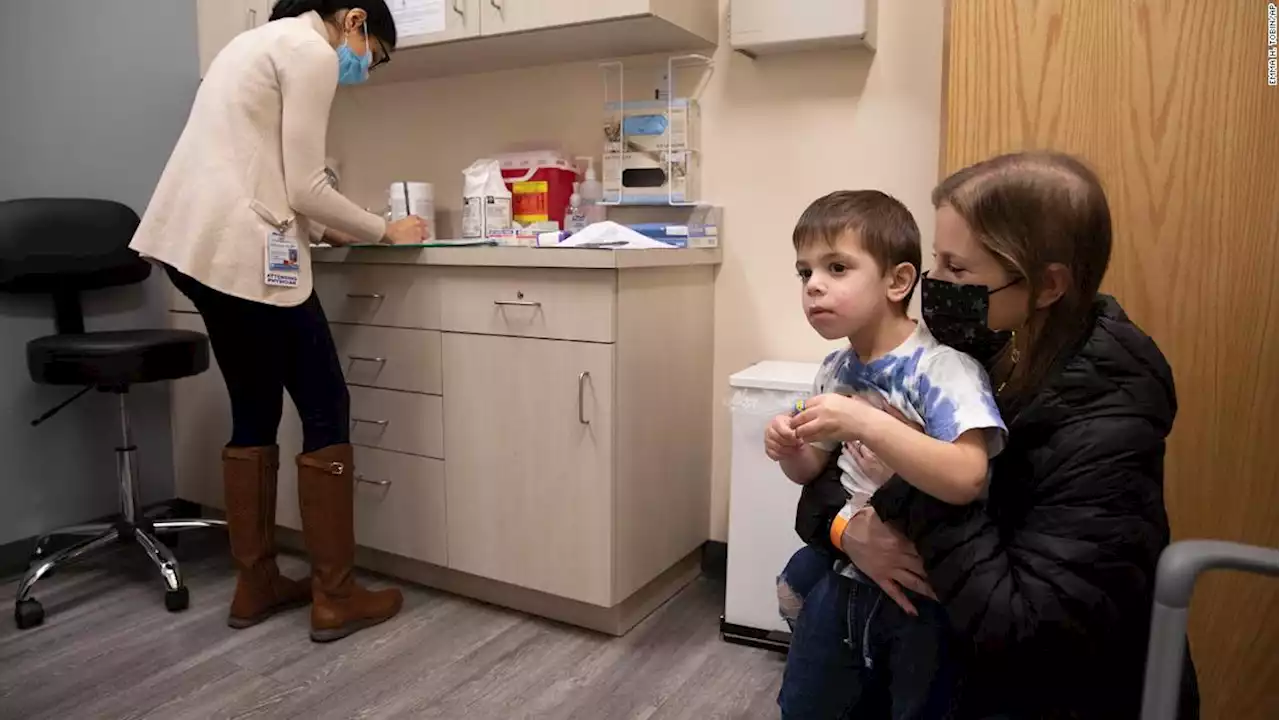 CDC advisers vote to recommend Covid-19 vaccines for children as young as 6 monthsVaccine advisers to the US Centers for Disease Control and Prevention voted unanimously on Saturday in support of recommending the Moderna and Pfizer/BioNTech Covid-19 vaccines to children as young as 6 months.
CDC advisers vote to recommend Covid-19 vaccines for children as young as 6 monthsVaccine advisers to the US Centers for Disease Control and Prevention voted unanimously on Saturday in support of recommending the Moderna and Pfizer/BioNTech Covid-19 vaccines to children as young as 6 months.
Leer más »
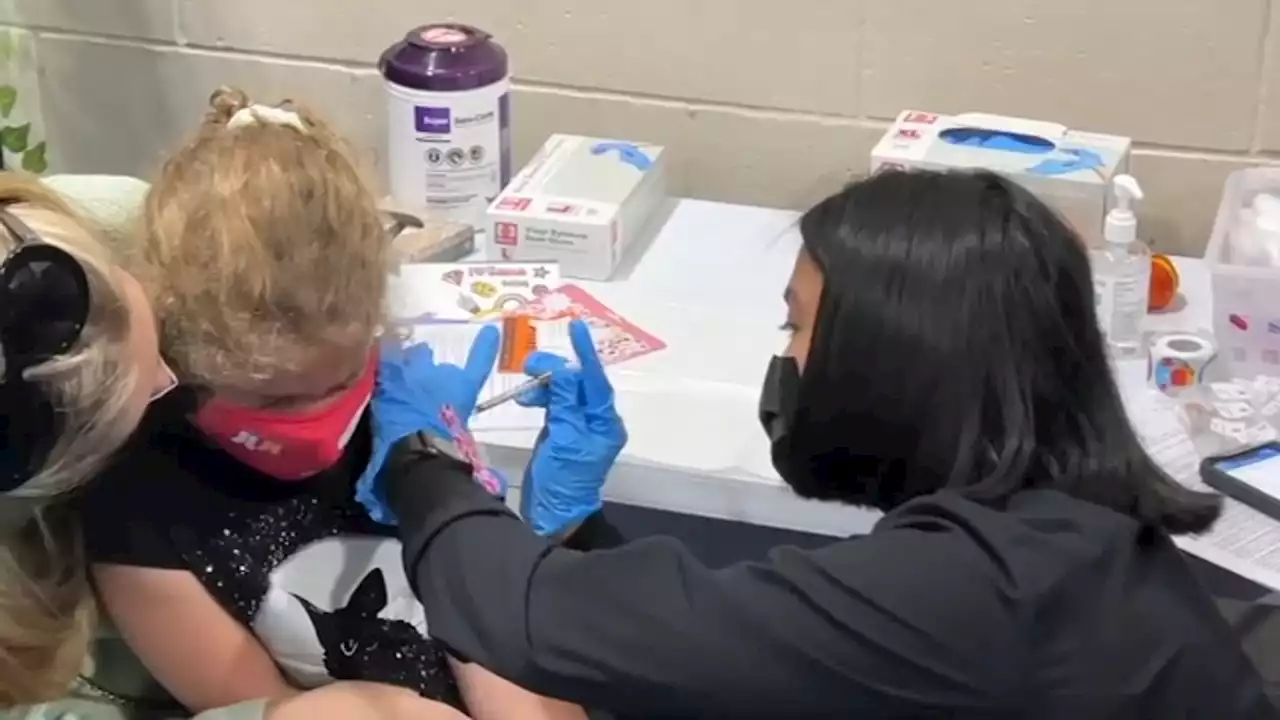 CDC panel meeting on COVID-19 vaccine for younger kidsThe next steps towards approving COVID vaccines for young children are underway and in the hands of the CDC.
CDC panel meeting on COVID-19 vaccine for younger kidsThe next steps towards approving COVID vaccines for young children are underway and in the hands of the CDC.
Leer más »
 CDC Advisers Reviewing Covid-19 Vaccines for Young ChildrenA recommendation from the advisers, who are meeting Friday and Saturday to review data around vaccinating those under 5 years old, could pave the way for expanding access to the shots.
CDC Advisers Reviewing Covid-19 Vaccines for Young ChildrenA recommendation from the advisers, who are meeting Friday and Saturday to review data around vaccinating those under 5 years old, could pave the way for expanding access to the shots.
Leer más »
 COVID-19 vaccine: CDC panel approves 1st shots for US kids under 5With the CDC's final sign-off, roughly 18 million children under 5 would be eligible for COVID-19 shots.
COVID-19 vaccine: CDC panel approves 1st shots for US kids under 5With the CDC's final sign-off, roughly 18 million children under 5 would be eligible for COVID-19 shots.
Leer más »
 FDA authorizes 1st COVID-19 shots for kids under 5; CDC review is nextU.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
FDA authorizes 1st COVID-19 shots for kids under 5; CDC review is nextU.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
Leer más »
