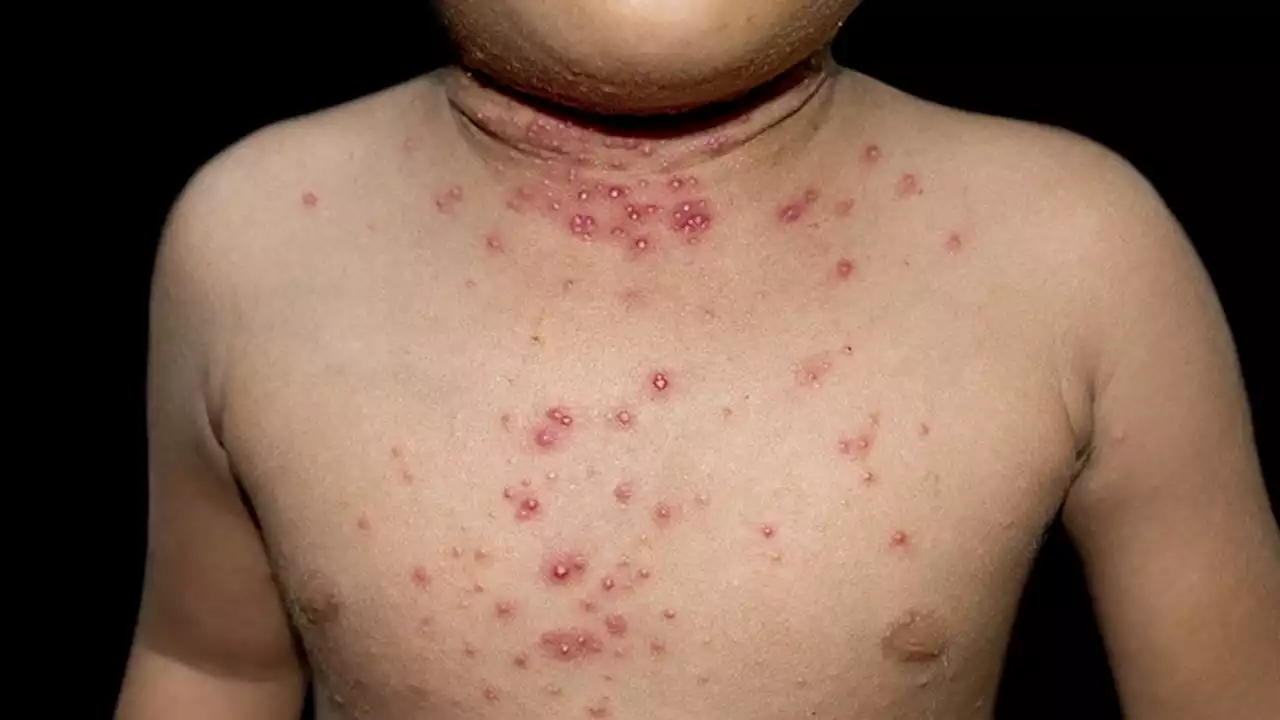
FDA accepts application for topical molluscum treatment. medtwitter FDA
The Food and Drug Administration has accepted a new drug application for berdazimer gel 10.3% for the treatment of molluscum contagiosum, the manufacturer If approved, berdazimer gel would be the first FDA-approved prescription product for molluscum contagiosum in the United States, according to the company, Novan. The active ingredient in berdazimer gel 10.3% is berdazimer sodium, a novel nitric oxide–releasing agent.
Molluscum contagiosum is a benign but contagious skin infection characterized by red papules on the face, trunk, limbs, and axillae that may persist for years if left untreated., a phase 3 clinical trial including 891 individuals with molluscum contagiosum aged 6 months and older, with 3-70 raised lesions The mean age of the patients was approximately 7 years and 85.5% were White . Study participants were randomized to berdazimer gel 10.
The Prescription Drug User Fee goal date for the approval of berdazimer 10.3% for molluscum contagiosum is set for Jan. 5, 2024, according to Novan.Lead image: Zay Nyi Nyi/Dreamstime
México Últimas Noticias, México Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 John Cena Eviscerates Austin Theory, Accepts WrestleMania ChallengeJohn Cena accepted a WrestleMania challenge from Austin Theory on WWE Raw last night, but Theory was already defeated before Cena left the ring.
John Cena Eviscerates Austin Theory, Accepts WrestleMania ChallengeJohn Cena accepted a WrestleMania challenge from Austin Theory on WWE Raw last night, but Theory was already defeated before Cena left the ring.
Leer más »
 FDA will review Eisai and Biogen's Alzheimer's treatment Leqembi with decision on full approval expected in early JulyFDA will review Eisai's and Biogen's Alzheimer's treatment Leqembi with decision on full approval expected in early July
FDA will review Eisai and Biogen's Alzheimer's treatment Leqembi with decision on full approval expected in early JulyFDA will review Eisai's and Biogen's Alzheimer's treatment Leqembi with decision on full approval expected in early July
Leer más »
 Justice Department launches application to seek pardon for federal marijuana convictionsPresident Joe Biden pardoned those with prior federal convictions last year, but some complained the busts were still showing up on their records. CannabisCommunity cannabisindustry cannabisculture CannabisNews JoeBiden
Justice Department launches application to seek pardon for federal marijuana convictionsPresident Joe Biden pardoned those with prior federal convictions last year, but some complained the busts were still showing up on their records. CannabisCommunity cannabisindustry cannabisculture CannabisNews JoeBiden
Leer más »
 Bruton Tyrosine Kinase Inhibitors for Chronic Lymphocytic Leukemia: Data, Application, and Patient Selection❗ Do you agree that Bruton tyrosine kinase inhibitors have significantly transformed the treatment of chronic lymphocytic leukemia? CLL MedEd CME ➡️
Bruton Tyrosine Kinase Inhibitors for Chronic Lymphocytic Leukemia: Data, Application, and Patient Selection❗ Do you agree that Bruton tyrosine kinase inhibitors have significantly transformed the treatment of chronic lymphocytic leukemia? CLL MedEd CME ➡️
Leer más »
 Hungarian delegation backs Sweden's NATO applicationCOPENHAGEN, Denmark (AP) — A parliamentary delegation from Hungary said Tuesday that it supports Sweden’s NATO membership bid after meeting the speaker of the Swedish parliament to iron out what Hungary’s governing party has called “political disputes.”
Hungarian delegation backs Sweden's NATO applicationCOPENHAGEN, Denmark (AP) — A parliamentary delegation from Hungary said Tuesday that it supports Sweden’s NATO membership bid after meeting the speaker of the Swedish parliament to iron out what Hungary’s governing party has called “political disputes.”
Leer más »
 Opinion | The Government’s Covid CasualtiesFrom WSJopinion: FDA delays carry human and financial costs, and examples are the fates of the Covid vaccine maker Novavax and the testing start-up Lucira
Opinion | The Government’s Covid CasualtiesFrom WSJopinion: FDA delays carry human and financial costs, and examples are the fates of the Covid vaccine maker Novavax and the testing start-up Lucira
Leer más »