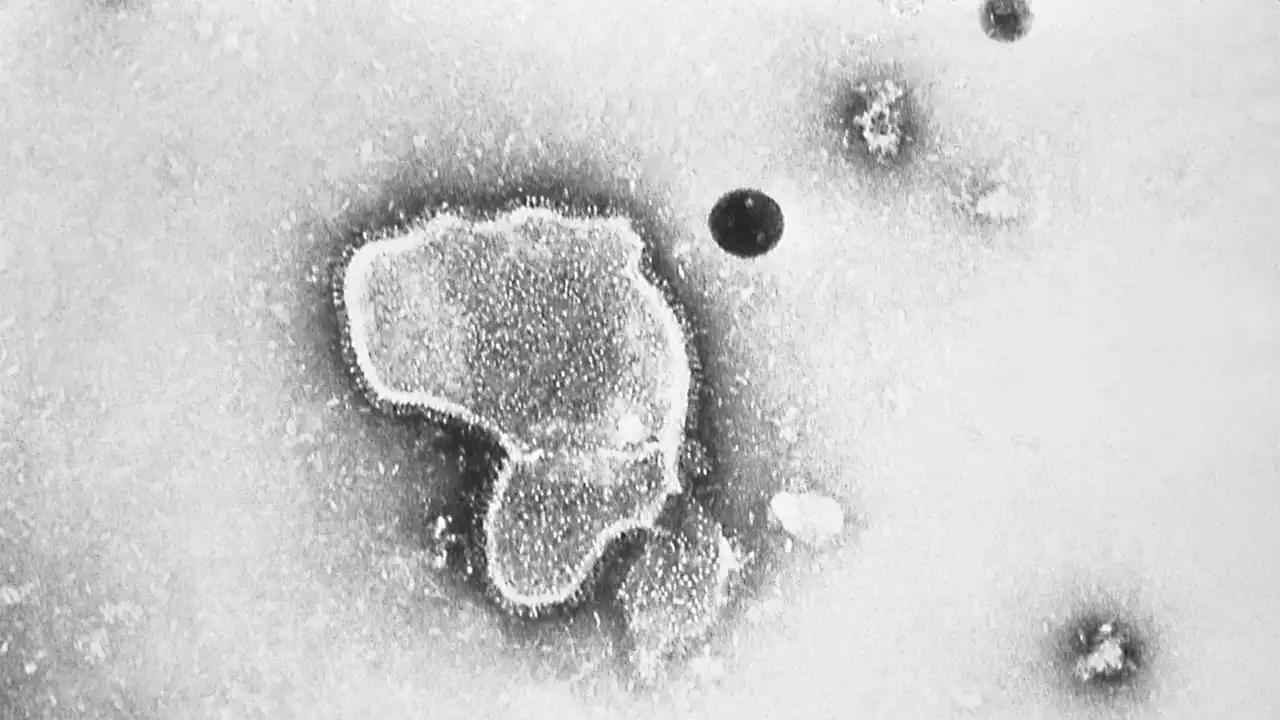
This injection was approved to protect against the annual leading cause of hospitalization in infants under a year old in the U.S.
Nirsevimab, which will be sold under the brand name Beyfortus, is not a vaccine. Vaccines prompt the body to make antibodies to defend against pathogens. Instead, nirsevimab is a form of passive immunity. It's a ready-made antibody that can bind to the virus and block it from infecting healthy cells. The immune system doesn't have to make anything.
After the CDC signs off, nirsevimab will become the second antibody available to protect young children against RSV. The other, called palivizumab or Synagis, has been used only to protect the most vulnerable babies -those born prematurely who are younger than 6 months. It lasts only short time in the body, so doctors give it once a month, starting just before RSV season, until the risk has passed.
That vaccine would protect babies from the moment they are born, a benefit if the infection shows up out of season. Vaccines also prompt the mom's body to make more than one kind of antibody, which would provide broader-spectrum protection. In the clinical trials that led to its approval, nirsevimab was about 70% effective at cutting the risk that a baby would need a doctor's visit for RSV, and it was about 78% effective at preventing hospitalizations due to RSV compared with a placebo, according to an FDA analysis.
México Últimas Noticias, México Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 New drug to protect babies and toddlers from RSV gets FDA approval ahead of cold seasonLast year, a surge in RSV cases flooded U.S. hospitals with wheezing children. There are no vaccines for babies yet, though Pfizer and other companies are working on them.
New drug to protect babies and toddlers from RSV gets FDA approval ahead of cold seasonLast year, a surge in RSV cases flooded U.S. hospitals with wheezing children. There are no vaccines for babies yet, though Pfizer and other companies are working on them.
Leer más »
 FDA approves new drug to protect babies from RSVThe FDA approved a new drug to protect babies from RSV, the leading cause of hospitalization for U.S. infants.
FDA approves new drug to protect babies from RSVThe FDA approved a new drug to protect babies from RSV, the leading cause of hospitalization for U.S. infants.
Leer más »
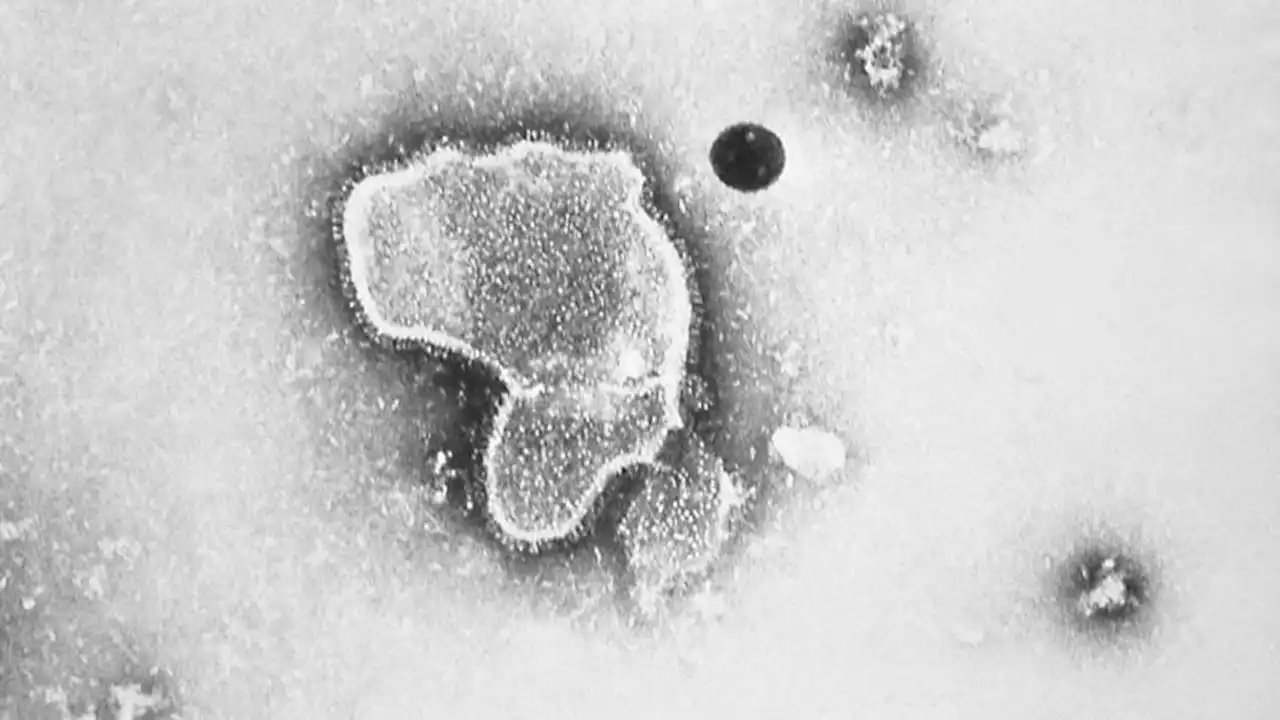 FDA approves antibody to protect infants from RSV | CNNParents and pediatricians will have a new option this fall to protect babies from a lung-attacking virus that is the leading cause of hospitalization in infants under a year of age in the United States every year.
FDA approves antibody to protect infants from RSV | CNNParents and pediatricians will have a new option this fall to protect babies from a lung-attacking virus that is the leading cause of hospitalization in infants under a year of age in the United States every year.
Leer más »
 FDA approves antibody shot to protect infants from potentially deadly virus RSVThis injection was approved to protect against the annual leading cause of hospitalization in infants under a year old in the U.S.
FDA approves antibody shot to protect infants from potentially deadly virus RSVThis injection was approved to protect against the annual leading cause of hospitalization in infants under a year old in the U.S.
Leer más »
 The Pill, Over the Counter | The Brian Lehrer Show | WNYCFor the first time, the FDA has approved over the counter sale of a birth control pill, called Opill.
The Pill, Over the Counter | The Brian Lehrer Show | WNYCFor the first time, the FDA has approved over the counter sale of a birth control pill, called Opill.
Leer más »
 Eli Lilly expects FDA decision on Alzheimer's treatment donanemab by the end of the yearEli Lilly says it has applied for full FDA approval of its Alzheimer’s treatment, donanemab, and expects the agency to make a decision by the end of the year. CEO David Ricks joins SquawkStreet to discuss.
Eli Lilly expects FDA decision on Alzheimer's treatment donanemab by the end of the yearEli Lilly says it has applied for full FDA approval of its Alzheimer’s treatment, donanemab, and expects the agency to make a decision by the end of the year. CEO David Ricks joins SquawkStreet to discuss.
Leer más »