The FDA has approved 'the world's first' leadless dual-chamber pacing system, one based in part on an already-approved leadless single-chamber device. MedicalNews CardioTwitter
The US Food and Drug Administration has approved"the world's first" leadless dual-chamber pacing system, one based in part on anThe company's AVEIR DR leadless pacing system consists of two percutaneously implanted devices, the single-chamber AVEIR VR leadless pacemaker, implanted within the right ventricle, and the novel AVEIR AR single-chamber pacemaker for implantation in the right atrium.
The AVEIR DR system relies on proprietary wireless technology to provide bi-directional, beat-to-beat communication between its two components to achieve dual-chamber synchronization, the company stated in a press release on the approval. The system also provides real-time pacing analysis, Abbott said, allowing clinicians to assess proper device placement during the procedure and before implantation. The system is designed to be easily removed if the patient's pacing needs evolve or its battery needs replacing.
Experienced operators achieved a 98% implantation success rate using the AVIER DR system in a 300-patient study conducted at 55 sites in Canada, Europe, and the United States, as
México Últimas Noticias, México Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 FDA Gives The Okay For Abbott’s New Minimally Invasive PacemakerOn Wednesday, medtech giant Abbott announced that its new leadless pacemaker system, Aveir DR, has been approved by the FDA. This is the first time the FDA has given a thumbs up to a device of this type for two different chambers of the heart.
FDA Gives The Okay For Abbott’s New Minimally Invasive PacemakerOn Wednesday, medtech giant Abbott announced that its new leadless pacemaker system, Aveir DR, has been approved by the FDA. This is the first time the FDA has given a thumbs up to a device of this type for two different chambers of the heart.
Leer más »
 FDA Gives The Okay For Abbott’s New Minimally Invasive PacemakerMedtech giant Abbott announced that its new leadless pacemaker system, Aveir DR, has been approved by the FDA.
FDA Gives The Okay For Abbott’s New Minimally Invasive PacemakerMedtech giant Abbott announced that its new leadless pacemaker system, Aveir DR, has been approved by the FDA.
Leer más »
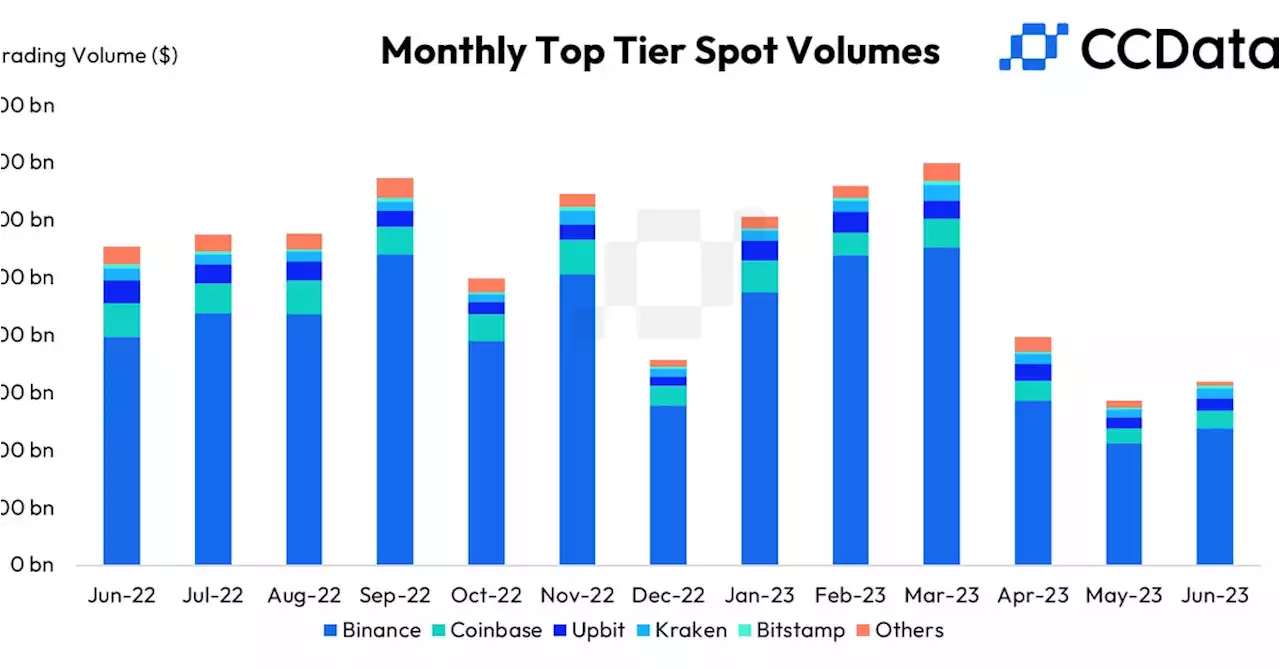 First Mover Americas: Cryptocurrency Trading Volumes Increase for First Time in Three MonthsCrypto trading volumes rose in June for the first time in three months amid optimism following the filing of spot bitcoin exchange-traded-fund (ETF) proposals by BlackRock and others. LedesmaLyllah and godbole17 report in First Mover Americas
First Mover Americas: Cryptocurrency Trading Volumes Increase for First Time in Three MonthsCrypto trading volumes rose in June for the first time in three months amid optimism following the filing of spot bitcoin exchange-traded-fund (ETF) proposals by BlackRock and others. LedesmaLyllah and godbole17 report in First Mover Americas
Leer más »
 FDA, FTC warn 6 companies allegedly selling delta-8 THC infused foods with confusing labelingThe FDA and Federal Trade Commission have issued warning letters to six companies it says are illegally selling copycat foods infused with delta-8 THC.
FDA, FTC warn 6 companies allegedly selling delta-8 THC infused foods with confusing labelingThe FDA and Federal Trade Commission have issued warning letters to six companies it says are illegally selling copycat foods infused with delta-8 THC.
Leer más »
 InnovationRx: FDA OKs Abbott’s New PacemakerInnovationRx is your weekly digest of healthcare news. Sign up!
InnovationRx: FDA OKs Abbott’s New PacemakerInnovationRx is your weekly digest of healthcare news. Sign up!
Leer más »
