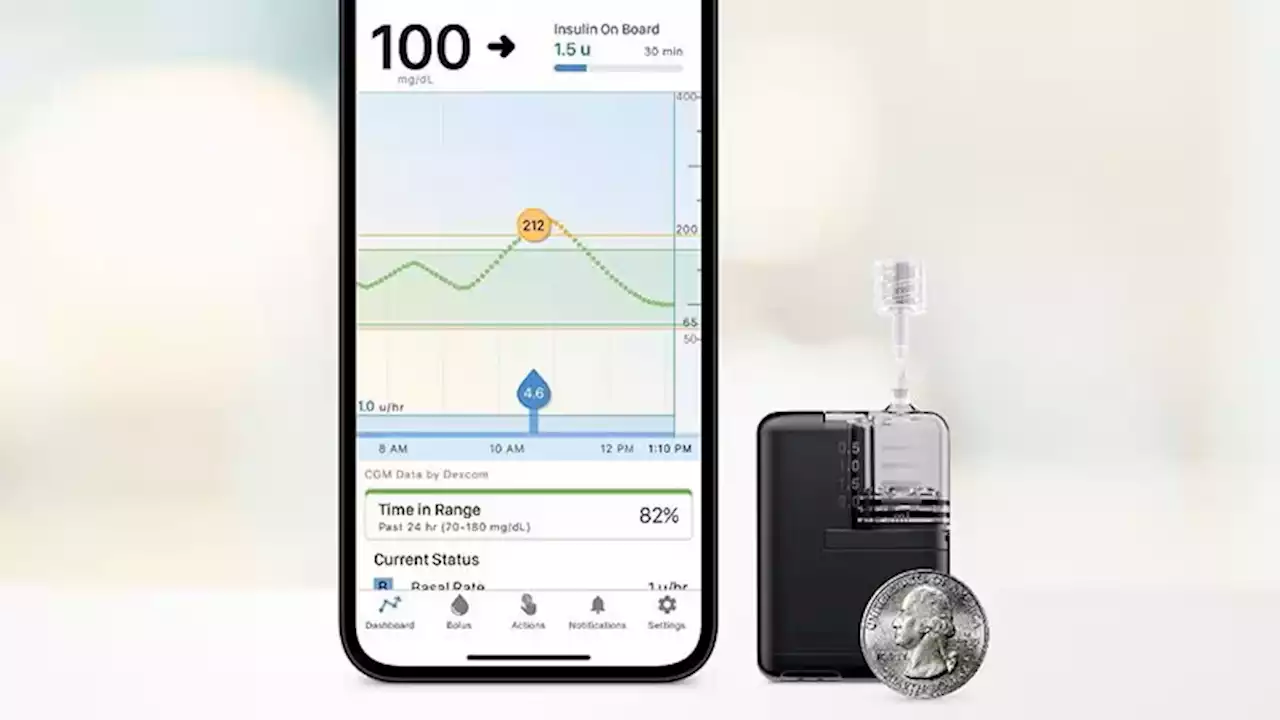
FDA clears Tandem Mobi insulin pump
The product is half the size of the company's t:slim X2 and is now the smallest of the commercially available durable tubed pumps. It is fully controllable from a mobile app through a user's compatible iPhone.cartridge and an on-pump button that can be used instead of the phone for bolusing insulin. The device can be clipped to clothing or worn on-body with an adhesive sleeve that is sold separately.
The Mobi is compatible with all existing Tandem-branded infusion sets manufactured by the Convatec Group, and there is a new 5-inch tubing option made just for the Tandem Mobi. The Mobi is part of a hybrid-closed loop automated delivery system, along with the current Control-IQ technology and a compatible continuous glucose monitor . The CGM sensor predicts glucose values 30 minutes ahead and adjusts insulin delivery every 5 minutes to prevent highs and lows. Users must still manually bolus for meals. The system can deliver automatic correction boluses for up to 1 hour to prevent hyperglycemia.
Limited release of the Tandom Mobi is expected in late 2023, followed by full commercial availability in early 2024. Miriam E. Tucker is a freelance journalist based in the Washington, DC area. She is a regular contributor to Medscape, with other work appearing in
México Últimas Noticias, México Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 Eleventh-hour deal clears path for Sweden to join NATO in rebuke to PutinA HISTORIC DAY: In recent weeks, NATO Secretary-General Jens Stoltenberg has demonstrated why member nations were so anxious to keep him around for another year. The former prime minister of Norway managed to broker a deal that clears the way for Sweden to join Finland as the newest member of the…
Eleventh-hour deal clears path for Sweden to join NATO in rebuke to PutinA HISTORIC DAY: In recent weeks, NATO Secretary-General Jens Stoltenberg has demonstrated why member nations were so anxious to keep him around for another year. The former prime minister of Norway managed to broker a deal that clears the way for Sweden to join Finland as the newest member of the…
Leer más »
 Sen. Charles Schumer presses FDA to look into Logan Paul’s PRIME energy drinkAn influencer-backed energy drink that has earned viral popularity among children is facing scrutiny from lawmakers and health experts over its potentially dangerous levels of caffeine.
Sen. Charles Schumer presses FDA to look into Logan Paul’s PRIME energy drinkAn influencer-backed energy drink that has earned viral popularity among children is facing scrutiny from lawmakers and health experts over its potentially dangerous levels of caffeine.
Leer más »
 Sen. Schumer asks FDA to look into PRIME, Logan Paul's high-caffeine energy drinkSen. Charles Schumer called on the Food and Drug Administration to investigate PRIME, a beverage brand founded by the YouTube stars Logan Paul and KSI, and to warn parents about the drink and the high amount of caffeine it contains.
Sen. Schumer asks FDA to look into PRIME, Logan Paul's high-caffeine energy drinkSen. Charles Schumer called on the Food and Drug Administration to investigate PRIME, a beverage brand founded by the YouTube stars Logan Paul and KSI, and to warn parents about the drink and the high amount of caffeine it contains.
Leer más »
 Schumer asks FDA to investigate Logan Paul's PRIME energy drinkU.S. Senate Majority Leader Chuck Schumer on Sunday called on regulators to investigate a popular influencer-created energy drink with nearly twice the caffeine of a Red Bull that he said is being marketed to children.
Schumer asks FDA to investigate Logan Paul's PRIME energy drinkU.S. Senate Majority Leader Chuck Schumer on Sunday called on regulators to investigate a popular influencer-created energy drink with nearly twice the caffeine of a Red Bull that he said is being marketed to children.
Leer más »
 Schumer asks FDA to investigate high caffeine levels in PRIME energy drinksPRIME contains more than six times the amount of caffeine found in a similar size of Coke.
Schumer asks FDA to investigate high caffeine levels in PRIME energy drinksPRIME contains more than six times the amount of caffeine found in a similar size of Coke.
Leer más »
 Schumer calls on FDA to investigate Logan Paul’s energy drinkSenate Majority Leader Chuck Schumer sent a letter to the Food and Drug Administration to urge the regulator to look into Prime energy drinks and their caffeine content.
Schumer calls on FDA to investigate Logan Paul’s energy drinkSenate Majority Leader Chuck Schumer sent a letter to the Food and Drug Administration to urge the regulator to look into Prime energy drinks and their caffeine content.
Leer más »