The FDA said it is the first approval for 'one of the most commonly prescribed complex drug-device combination products to treat asthma and COPD.'
“Today’s approval of the first generic for one of the most commonly prescribed complex drug-device combination products to treat asthma and COPD is another step forward in our commitment to bring generic copies of complex drugs to the market, which can improve quality of life and help reduce the cost of treatment,” said Sally Choe, Ph.D., director of the Office of Generic Drugs in the FDA Center for Drug Evaluation and Research.
According to the FDA, 25 million people are affected by asthma while 16 million people have COPD - chronic obstructive pulmonary disease. The FDA said approval for the metered-dose inhaler was granted to Mylan Pharmaceuticals. The inhaler contains “budesonide and formoterol .” Two inhalations a day, the FDA said, treats both diseases by preventing symptoms. It is approved for two strengths.
Note to readers: if you purchase something through one of our affiliate links we may earn a commission.
México Últimas Noticias, México Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 FDA approves first Symbicort generic drug for asthma and COPDThe drug is an inhalation aerosol that treats symptoms of two common pulmonary health conditions.
FDA approves first Symbicort generic drug for asthma and COPDThe drug is an inhalation aerosol that treats symptoms of two common pulmonary health conditions.
Leer más »
 3 COVID-19 tests recalled after FDA warns about ‘high risk’ of false resultsLuSys Laboratories issued the recall this week for three of its COVID-19 tests. The FDA believes they were distributed for use in laboratories and for at-home testing.
3 COVID-19 tests recalled after FDA warns about ‘high risk’ of false resultsLuSys Laboratories issued the recall this week for three of its COVID-19 tests. The FDA believes they were distributed for use in laboratories and for at-home testing.
Leer más »
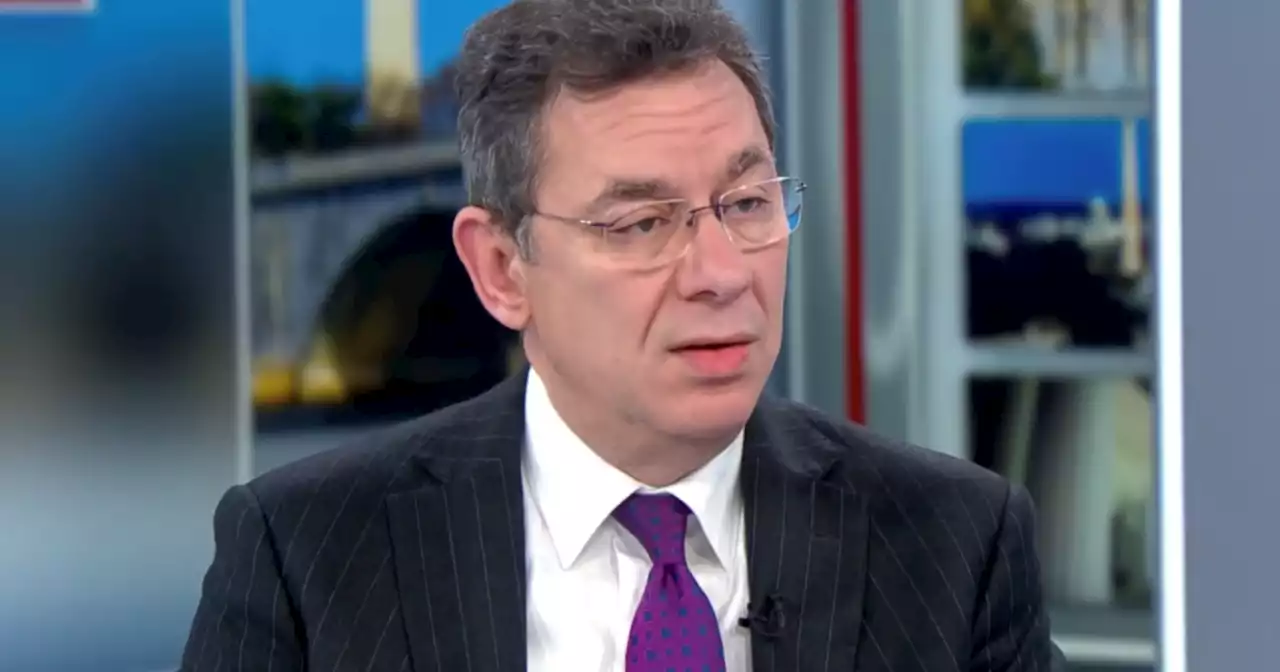 Pfizer CEO says fourth dose of COVID vaccine is 'necessary,' submits data to FDACEO Albert Bourla said the vaccine remains protective against hospitalizations and deaths, but 'doesn't last very long' for warding off infections.
Pfizer CEO says fourth dose of COVID vaccine is 'necessary,' submits data to FDACEO Albert Bourla said the vaccine remains protective against hospitalizations and deaths, but 'doesn't last very long' for warding off infections.
Leer más »
 How the FDA bungled the powdered infant formula recallA manufacturing plant that produced baby formula had a long history of safety regulation violations. Yet it remained open, and it was only after a baby died that the company issued a recall.
How the FDA bungled the powdered infant formula recallA manufacturing plant that produced baby formula had a long history of safety regulation violations. Yet it remained open, and it was only after a baby died that the company issued a recall.
Leer más »
 Pfizer/BioNTech seek FDA authorization for fourth Covid-19 vaccine doses for people 65 and upPfizer and BioNTech submitted an application for emergency use authorization to the US Food and Drug Administration for an additional booster dose of their Covid-19 vaccine for adults 65 and older who have gotten a booster dose of any of the authorized or approved vaccine, the companies said Tuesday.
Pfizer/BioNTech seek FDA authorization for fourth Covid-19 vaccine doses for people 65 and upPfizer and BioNTech submitted an application for emergency use authorization to the US Food and Drug Administration for an additional booster dose of their Covid-19 vaccine for adults 65 and older who have gotten a booster dose of any of the authorized or approved vaccine, the companies said Tuesday.
Leer más »