The FDA has authorized bivalent COVID-19 vaccines for children as young as 6 months old.
Children ages 4 and under who have already completed their three-dose Pfizer vaccine series won’t be eligible for boosters until next year, when more data is available, the FDA said in a“Children in this age group who already completed their primary [Pfizer] series would still be expected to have protection against the most serious outcomes from the currently circulating omicron variant,” the news release stated.
The term"bivalent" means the new vaccines are updated to address virus strains that have become more common since the primary vaccine formula was developed.
México Últimas Noticias, México Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 FDA authorizes updated COVID shots for kids as young as 6 months oldThe FDA is encouraging families to get young children vaccinated ahead of the holidays.
FDA authorizes updated COVID shots for kids as young as 6 months oldThe FDA is encouraging families to get young children vaccinated ahead of the holidays.
Leer más »
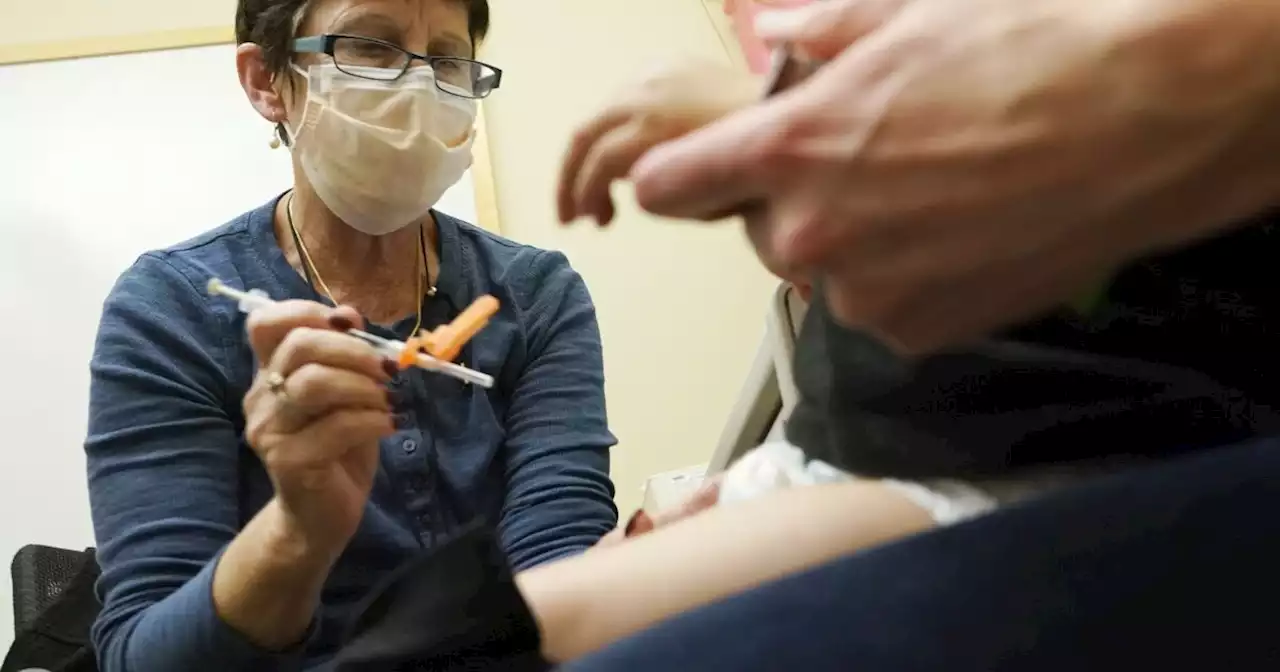 FDA clears updated COVID-19 vaccines for kids under age 5U.S. regulators have cleared doses of the updated COVID-19 vaccines to children younger than 5
FDA clears updated COVID-19 vaccines for kids under age 5U.S. regulators have cleared doses of the updated COVID-19 vaccines to children younger than 5
Leer más »
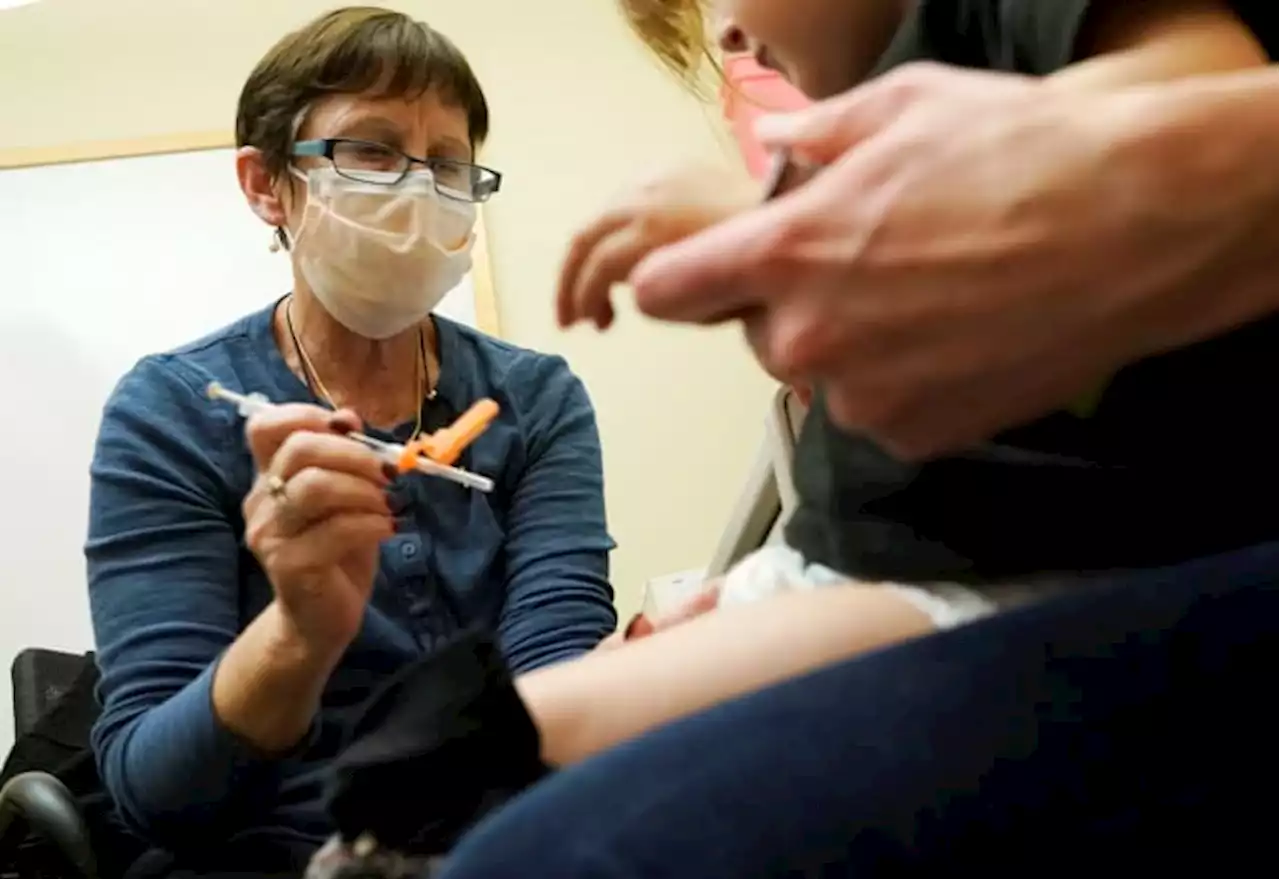 FDA clears updated COVID-19 vaccines for kids under age 5U.S. regulators on Thursday cleared doses of the updated COVID-19 vaccines for children younger than age 5.
FDA clears updated COVID-19 vaccines for kids under age 5U.S. regulators on Thursday cleared doses of the updated COVID-19 vaccines for children younger than age 5.
Leer más »
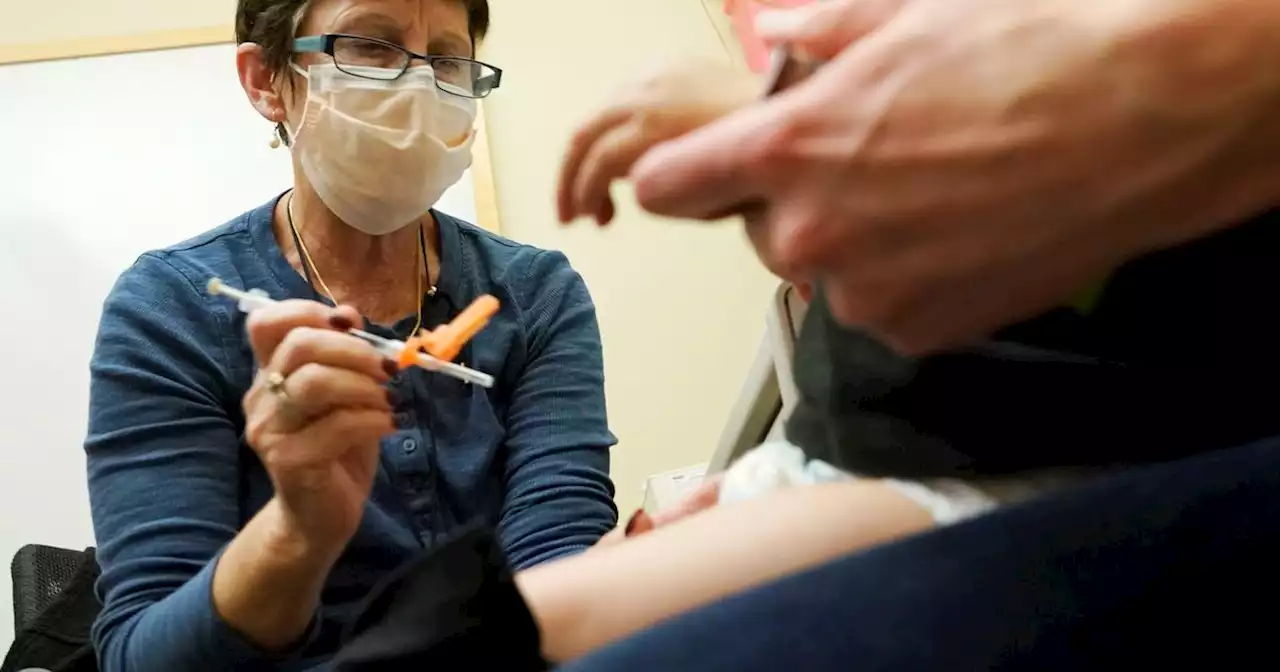 FDA clears updated COVID-19 vaccines for kids under age 5U.S. regulators on Thursday cleared doses of the updated COVID-19 vaccines for children younger than age 5. The Food and Drug Administration’s decision aims...
FDA clears updated COVID-19 vaccines for kids under age 5U.S. regulators on Thursday cleared doses of the updated COVID-19 vaccines for children younger than age 5. The Food and Drug Administration’s decision aims...
Leer más »
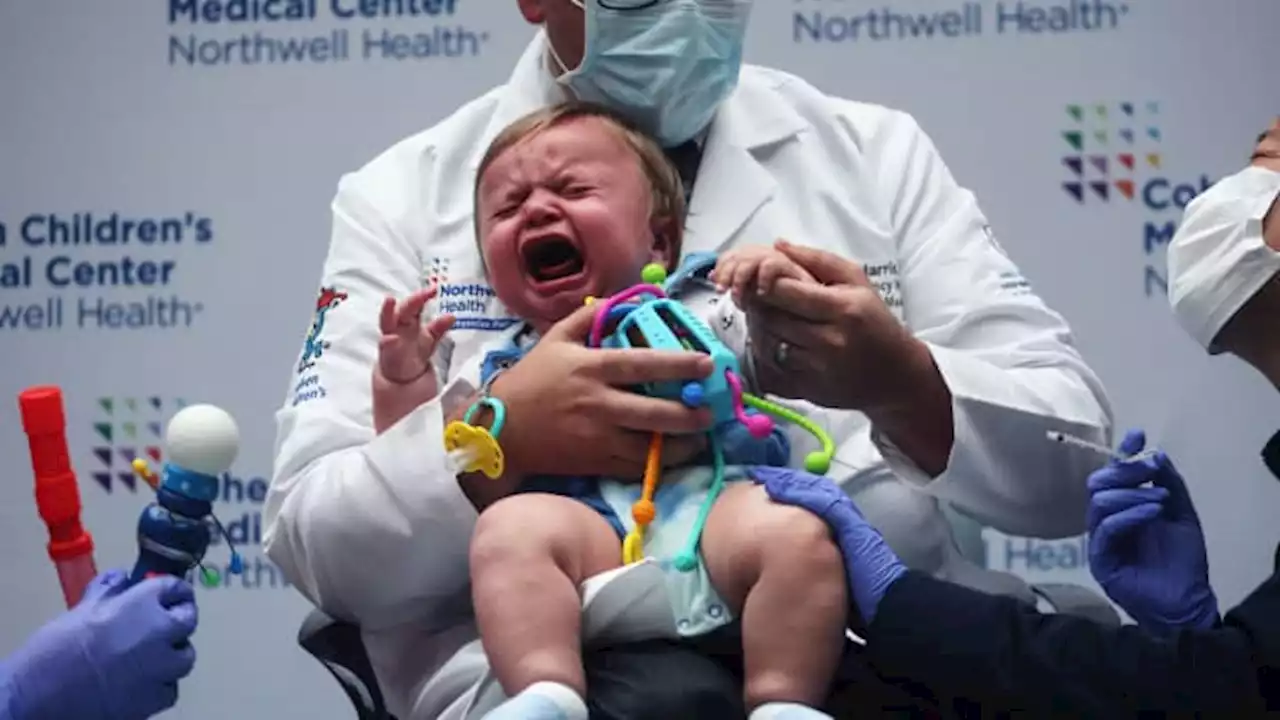 FDA authorizes Covid omicron vaccines for children as young as 6 months old
FDA authorizes Covid omicron vaccines for children as young as 6 months old
Leer más »
