A federal advisory committee recommended that a fourth COVID-19 vaccine be authorized for use in the USA, this one from Novavax.
Americans may soon get a new COVID-19 vaccine option — a more traditional kind of shot known as a protein vaccine. It's late in the pandemic for a new choice, but Novavax is hoping to find a niche with those who are unvaccinated or need boosters. recommended Tuesday that a fourth COVID-19 vaccine be authorized for use in the USA, this one from Novavax, a company based in Gaithersburg, Maryland.
Novavax said it has millions of doses available and ready to be shipped once it receives authorization. Coronavirus variants have emerged since the vaccine was tested, but Novavax officials said they are confident the vaccine will be effective. The company is prepared to"pivot" to protect against other variants as they emerge, according to the statement.
The Novavax vaccine is a protein-based vaccine and includes an adjuvant to boost its effectiveness. The protein-based technology has been used in other vaccines.
México Últimas Noticias, México Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 Novavax hoping its COVID-19 vaccine using traditional technology wins over vaccine skepticsThe Novavax COVID-19 vaccine is made with an old school technology, but will it be enough to win over vaccine skeptics?
Novavax hoping its COVID-19 vaccine using traditional technology wins over vaccine skepticsThe Novavax COVID-19 vaccine is made with an old school technology, but will it be enough to win over vaccine skeptics?
Leer más »
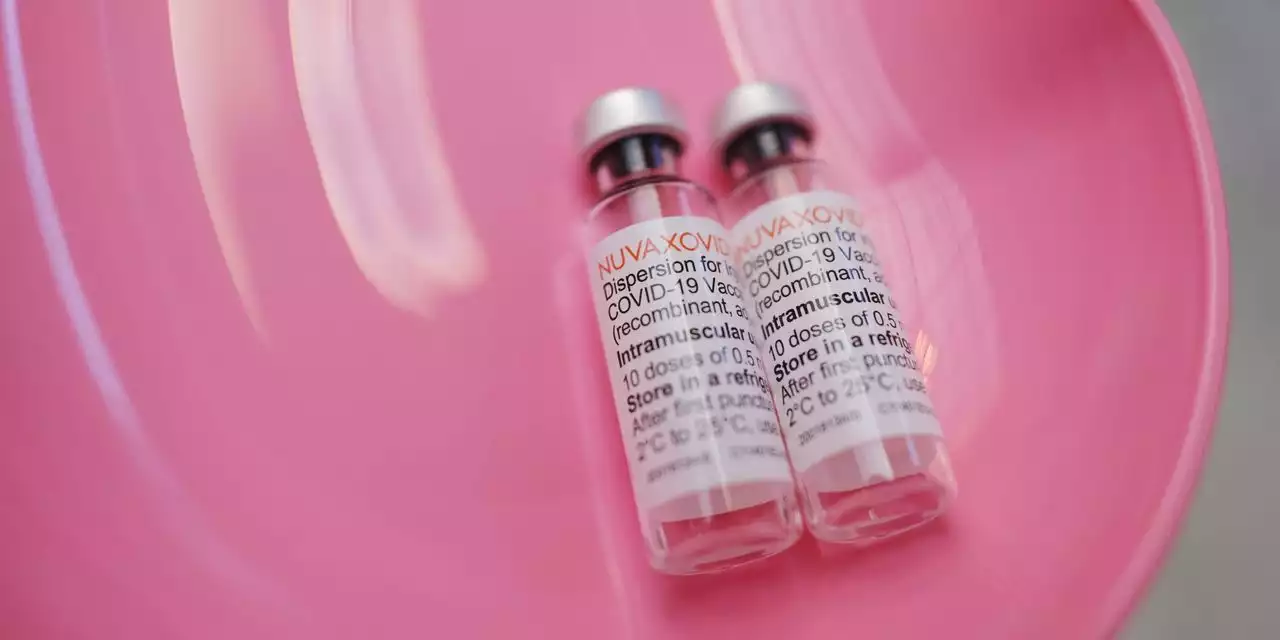 Novavax Covid-19 Vaccine Faces FDA Advisers’ ScrutinyThe outside panel of experts will vote on whether the shot should be authorized, after the agency found it effective but flagged a safety concern.
Novavax Covid-19 Vaccine Faces FDA Advisers’ ScrutinyThe outside panel of experts will vote on whether the shot should be authorized, after the agency found it effective but flagged a safety concern.
Leer más »
 FDA advisers meeting on Novavax, a latecomer in COVID-19 vaccine raceA federal advisory committee will vote on whether regulators should authorize a COVID-19 vaccine made by Novavax. From The New York Times.
FDA advisers meeting on Novavax, a latecomer in COVID-19 vaccine raceA federal advisory committee will vote on whether regulators should authorize a COVID-19 vaccine made by Novavax. From The New York Times.
Leer más »
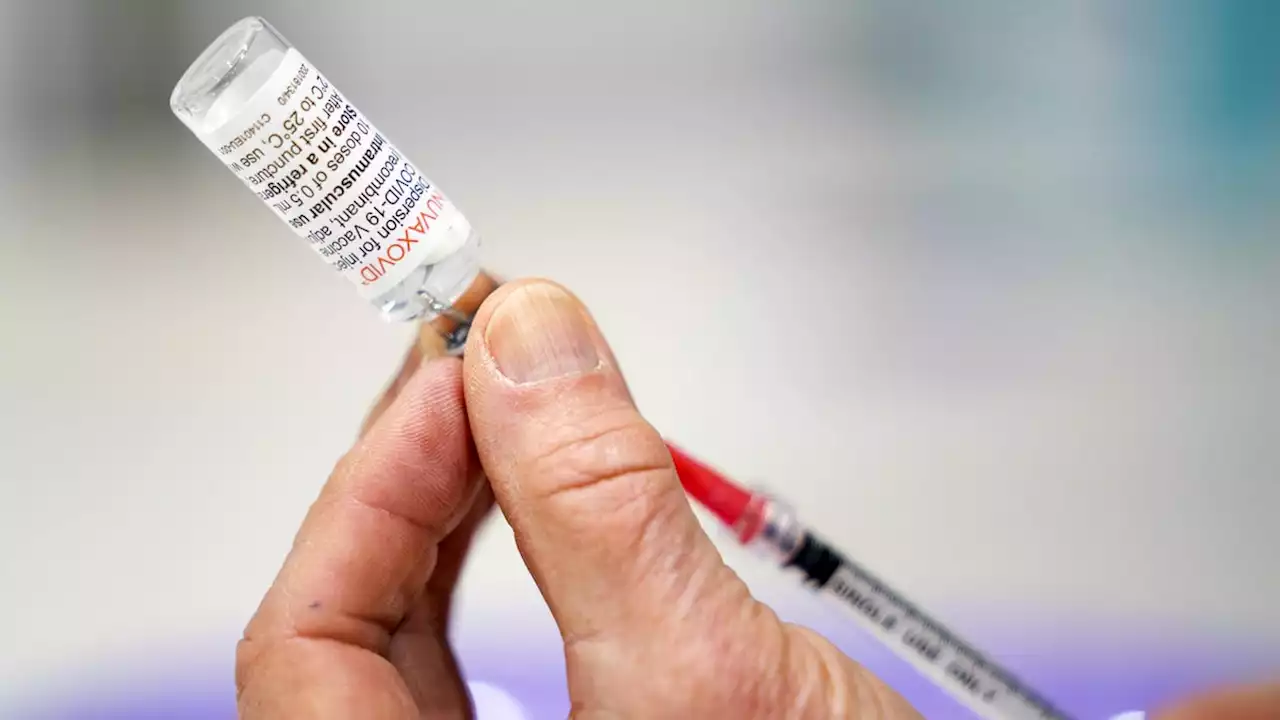 FDA advisory panel to consider approving fourth COVID-19 vaccine, this one from Maryland's NovavaxDevelopment of the vaccine, from Novavax, a Gaithersburg, Maryland-based company, was started at the same time as others in 2020, but the company struggled to produce its shot in large quantities.
FDA advisory panel to consider approving fourth COVID-19 vaccine, this one from Maryland's NovavaxDevelopment of the vaccine, from Novavax, a Gaithersburg, Maryland-based company, was started at the same time as others in 2020, but the company struggled to produce its shot in large quantities.
Leer más »
 FDA advisers back Novavax COVID-19 shots as new US optionNovavax shots are already used in Australia, Canada, parts of Europe and dozens of other countries. But U.S. clearance is a key hurdle for the Maryland-based company.
FDA advisers back Novavax COVID-19 shots as new US optionNovavax shots are already used in Australia, Canada, parts of Europe and dozens of other countries. But U.S. clearance is a key hurdle for the Maryland-based company.
Leer más »
 FDA panel greenlights Novavax COVID-19 vaccineFDA's vaccine chief said another choice in the U.S. may entice at least some vaccine holdouts - whatever their reason - to consider rolling up their sleeves. FOX13
FDA panel greenlights Novavax COVID-19 vaccineFDA's vaccine chief said another choice in the U.S. may entice at least some vaccine holdouts - whatever their reason - to consider rolling up their sleeves. FOX13
Leer más »