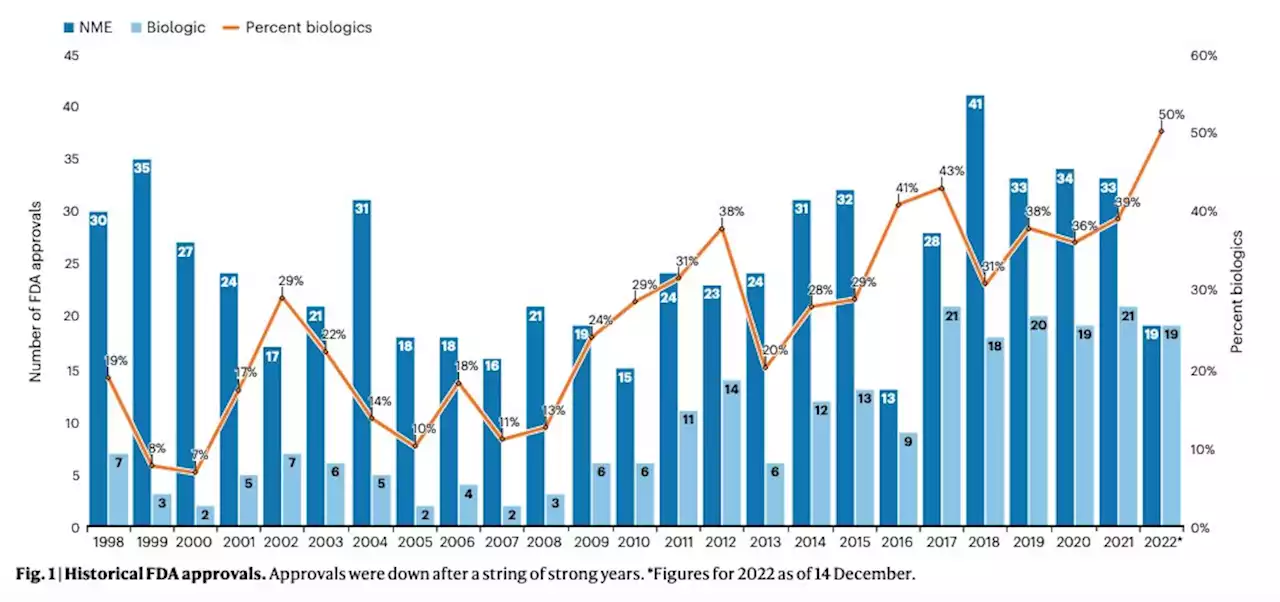
Fresh from the biotech pipeline: fewer approvals, but biologics gain share
by the US Department of Health and Human Services found that almost 40% of sponsors of the 278 drugs approved between 1992 and 2021 hadn’t completed them.
The FDA’s tightening appears to be deterring Accelerated Approval submissions. ADC Therapeutics said it wouldn’t pursue one for its Hodgkin’s lymphoma drug camidanlumab tesirine because the FDA had required a phase 3 program be planned and underway before the review started. Section 506 of the Federal Food, Drug and Cosmetic Act allows the FDA to make such a request, but the wording of the procedural guidance leaves wiggle room, which FDA officials wanted to limit.
Another force driving biologics’ growth is their evolution beyond simple antibodies, peptides or enzymes. Newer modalities including bispecific proteins, antibody–drug conjugates, and cell or gene therapies accounted for about half of biologic approvals through the end of November 2022, up from less than a third in 2021.
Carvykti became the sixth CAR-T cell therapy in the United States . The CAR-T cell R&D pipeline is huge, including sponsors seeking more convenient allogeneic varieties and ‘point of care’ manufacturing facilities to expand access, as well as those seeking to address solid tumors.
CSL Behring and licensee UniQure, based in Amsterdam, took the title for the most expensive drug in November with their $3.5 million price tag on Hemgenix , the first FDA-approved gene therapy for hemophilia B. Patients with this bleeding disorder lack clotting factor IX and require regular blood transfusions. Hemgenix uses an adeno-associated virus vector to deliver a factor IX gene to eligible patients on prophylaxis therapy or at risk of hemorrhage or spontaneous bleeding.
Yet regulators everywhere need time to properly investigate and understand the tools, approaches and manufacturing requirements underpinning many new medicines. The FDA in August 2022 put the brakes on some next-generation gene editing programs, including BEAM Therapeutics’ BEAM-201, an anti-CD7 multiplex-edited allogeneic CAR-T cell program for advanced T cell acute lymphoblastic leukemia and lymphoma.
Pluvicto is the first FDA approval of a targeted radioligand since Novartis’s Lutathera in 2018 for gastroenteropancreatic neuroendocrine tumors . Unconjugated radiotherapeutics had arrived five years earlier, with Leverkusen, Germany-based Bayer’s prostate cancer treatment Xofigo . Xofigo doesn’t include a separate ligand-targeting molecule; the radium itself, which is chemically like calcium, is attracted to actively growing bone tissue such as prostate cancer bone metastases.
An application from Lilly and Jiangsu, China-based Innovent Biologics for PD-1 inhibitor sintilimab was stopped in March 2022 because its China-only NSCLC trial was deemed not generalizable to the US population owing in part to the chemotherapy comparator. Sintilimab is sold in China as Tyvyt for Hodgkin’s disease, and Lilly had promised to offer the drug at a steep discount in the United States.
One aspect of trial diversity and representativeness may have far greater repercussions across drug R&D. Obesity now affects over 40% of the US adult population, and even more in some ethnic groups, according to the Centers for Disease Control and Prevention. Yet patients with obesity are routinely excluded from trials. “The implications of obesity cannot be overstated. Yet there’s a deficit of evidence about medicines in obese patients,” said the FDA’s Califf on November 9.
Progress in the quest for new drugs to combat obesity continued in 2022 with the arrival of Lilly’s dual glucagon-like peptide-1 /glucose-dependent insulinotropic polypeptide agonist Mounjaro . The drug was approved for type 2 diabetes but is also fast-tracked for obesity, in which Lilly plans to initiate a rolling submission before year end.
México Últimas Noticias, México Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 Unidirectional single-file transport of full-length proteins through a nanopore - Nature BiotechnologyFull-length, unfolded proteins are slowly translocated through nanopores without enzymes and fingerprinted.
Unidirectional single-file transport of full-length proteins through a nanopore - Nature BiotechnologyFull-length, unfolded proteins are slowly translocated through nanopores without enzymes and fingerprinted.
Leer más »
 Improved cytosine base editors generated from TadA variants - Nature BiotechnologyImproved cytosine base editors are generated by directed evolution of an adenosine deaminase.
Improved cytosine base editors generated from TadA variants - Nature BiotechnologyImproved cytosine base editors are generated by directed evolution of an adenosine deaminase.
Leer más »
 Ferrari 296 GTB 2022 review – fewer cylinders, greater thrills | EvoFerrari's first V6-powered road car redefines the £200,000 supercar with blistering pace and an entertaining chassis
Ferrari 296 GTB 2022 review – fewer cylinders, greater thrills | EvoFerrari's first V6-powered road car redefines the £200,000 supercar with blistering pace and an entertaining chassis
Leer más »
 Programmable A-to-Y base editing by fusing an adenine base editor with an N-methylpurine DNA glycosylase - Nature BiotechnologyProgrammable A-to-Y base editing by fusing an adenine base editor with an N-methylpurine DNA glycosylase
Programmable A-to-Y base editing by fusing an adenine base editor with an N-methylpurine DNA glycosylase - Nature BiotechnologyProgrammable A-to-Y base editing by fusing an adenine base editor with an N-methylpurine DNA glycosylase
Leer más »
 Cell-type-specific prediction of 3D chromatin organization enables high-throughput in silico genetic screening - Nature BiotechnologyDeep learning predicts cell-type-specific 3D chromatin contacts from DNA sequence, chromatin accessibility and CTCF binding.
Cell-type-specific prediction of 3D chromatin organization enables high-throughput in silico genetic screening - Nature BiotechnologyDeep learning predicts cell-type-specific 3D chromatin contacts from DNA sequence, chromatin accessibility and CTCF binding.
Leer más »
 Best of 2022: Spencer Legacy’s Top 10 TV Shows of the YearThere were quite a few brilliant TV series in 2022, so here are the ones that really stood out as the best of the best.-
Best of 2022: Spencer Legacy’s Top 10 TV Shows of the YearThere were quite a few brilliant TV series in 2022, so here are the ones that really stood out as the best of the best.-
Leer más »