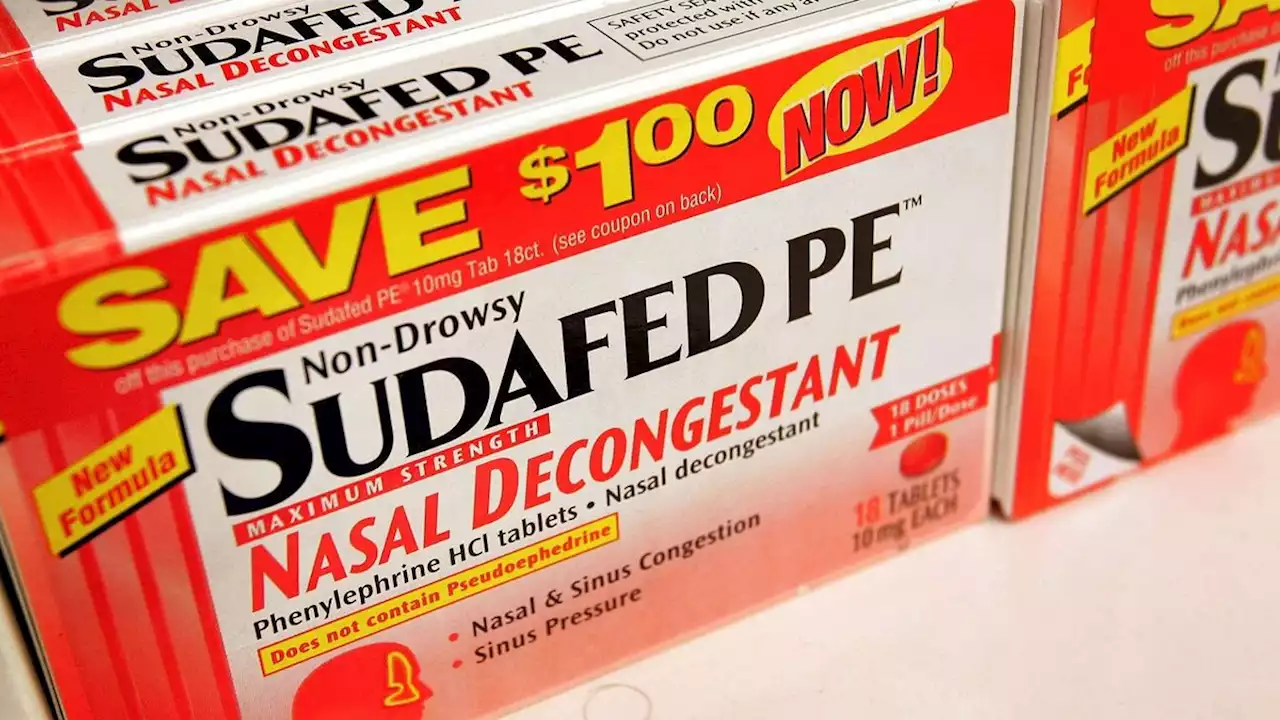
The Food and Drug Administration reviewed data on phenylephrine, an ingredient in many popular decongestants, and deemed it ineffective when taken orally.
The key ingredient in popular decongestants such as Sudafed PE, Benadryl Allergy Plus Congestion and Allegra-D doesn't relieve nasal congestion when taken orally, a Food and Drug Administration panel concluded in a meeting Tuesday .
That's because, when taken orally, less than 1% of the drug actually ends up in the bloodstream and thus reaches the tissues of the nose that it's supposed to help, the committee reported in a memo from the meeting. So, if they don't work, how did phenylephrine-based pills get approved in the first place?
Phenylephrine was first evaluated as an over-the-counter oral and intranasal decongestant back in 1976, according to the NDAC memo. But the ingredient gained popularity in 2005 as a substitute for pseudoephedrine, a different decongestant that had been moved behind the counter by a law intended to rein in the sale of drugs that can be used to make meth.
"These three trials represent by far the largest and most carefully constructed trials that have ever been performed to evaluate the decongestant effect of oral PE [phenylephrine]," the NDAC memo states. The trials showed that the drug had no more effect than a placebo, and additional data from the FDA's clinical pharmacology lab showed very little of it enters the bloodstream.
México Últimas Noticias, México Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 When Should You Get the New COVID Booster?The Food and Drug Administration (FDA) approved a new round of COVID boosters on Monday.
When Should You Get the New COVID Booster?The Food and Drug Administration (FDA) approved a new round of COVID boosters on Monday.
Leer más »
 FDA panel says common over-the-counter decongestant doesn’t workThe drug, called phenylephrine, is found in a number of over-the-counter cold and allergy medications.
FDA panel says common over-the-counter decongestant doesn’t workThe drug, called phenylephrine, is found in a number of over-the-counter cold and allergy medications.
Leer más »
 Popular nasal decongestant doesn't actually relieve congestion, FDA experts sayGovernment advisers say the leading decongestant used by millions of Americans to treat nasal congestion doesn't actually work.
Popular nasal decongestant doesn't actually relieve congestion, FDA experts sayGovernment advisers say the leading decongestant used by millions of Americans to treat nasal congestion doesn't actually work.
Leer más »
 Popular nasal decongestant doesn't actually relieve congestion, FDA experts sayGovernment advisers say the leading decongestant used by millions of Americans to treat nasal congestion doesn't actually work.
Popular nasal decongestant doesn't actually relieve congestion, FDA experts sayGovernment advisers say the leading decongestant used by millions of Americans to treat nasal congestion doesn't actually work.
Leer más »
 Popular nasal decongestant doesn't actually relieve congestion, FDA experts sayGovernment advisers say the leading decongestant used by millions of Americans to treat nasal congestion doesn't actually work.
Popular nasal decongestant doesn't actually relieve congestion, FDA experts sayGovernment advisers say the leading decongestant used by millions of Americans to treat nasal congestion doesn't actually work.
Leer más »
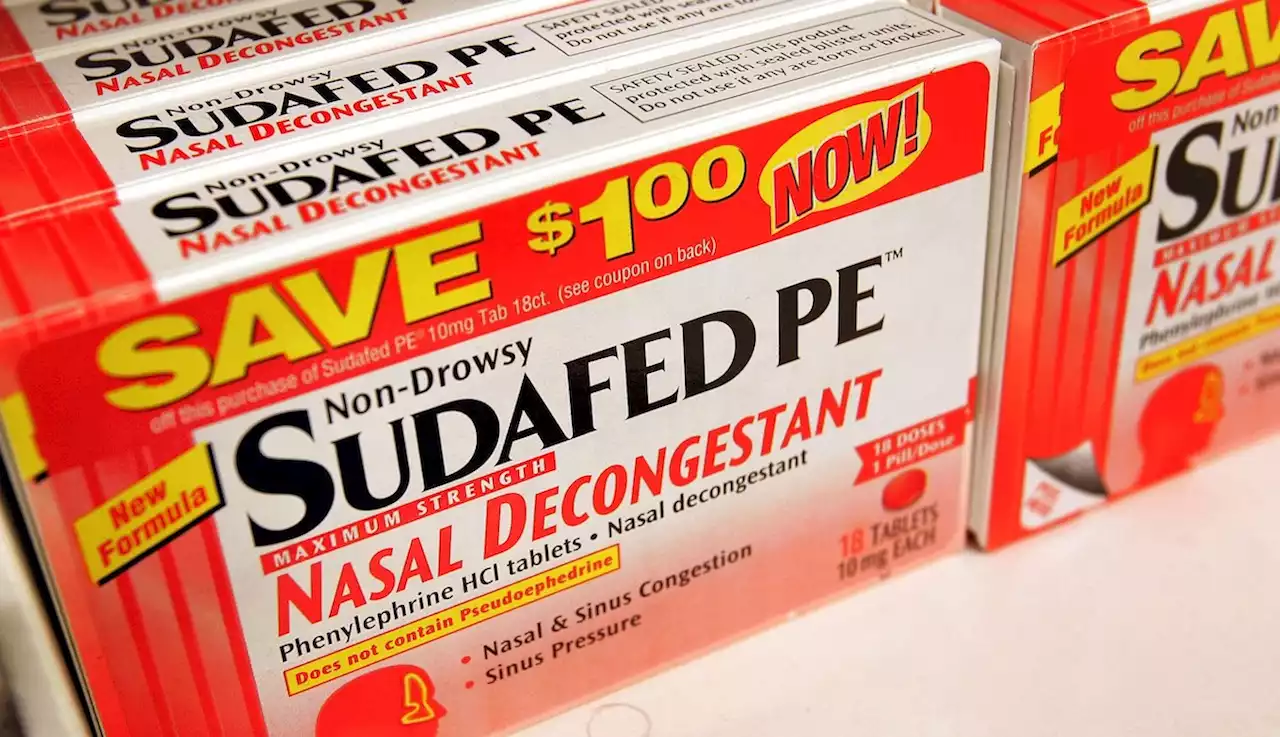 FDA panel says this popular over-the-counter decongestant doesn't actually workIf the FDA follows through on the panel’s recommendations, Johnson & Johnson, Bayer and other drugmakers could be required to pull their oral medications containing phenylephrine from store shelves.
FDA panel says this popular over-the-counter decongestant doesn't actually workIf the FDA follows through on the panel’s recommendations, Johnson & Johnson, Bayer and other drugmakers could be required to pull their oral medications containing phenylephrine from store shelves.
Leer más »