The request was described as much broader than that of Pfizer and BioNTech which was filed with the FDA earlier in the week.
Drugmaker Moderna has asked the Food and Drug Administration to authorize a fourth shot of its COVID-19 vaccine as a booster dose for all adults.
In a press release, the company says its request for approval for all adults was made “to provide flexibility” to the Centers for Disease Control and Prevention and medical providers to determine the “appropriate use” of a second booster dose of the mRNA vaccine, “including for those at higher risk of COVID-19 due to age or comorbidities.”
México Últimas Noticias, México Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 Moderna seeks FDA authorization for 4th dose of COVID shotJUST IN: Drugmaker Moderna asked the Food and Drug Administration on Thursday to authorize a fourth shot of its COVID-19 vaccine as a booster dose for all adults.
Moderna seeks FDA authorization for 4th dose of COVID shotJUST IN: Drugmaker Moderna asked the Food and Drug Administration on Thursday to authorize a fourth shot of its COVID-19 vaccine as a booster dose for all adults.
Leer más »
 Moderna seeks FDA authorization for 4th dose of COVID shotDrugmaker Moderna asked the Food and Drug Administration on Thursday to authorize a fourth shot of its COVID-19 vaccine as a booster dose for all adults.
Moderna seeks FDA authorization for 4th dose of COVID shotDrugmaker Moderna asked the Food and Drug Administration on Thursday to authorize a fourth shot of its COVID-19 vaccine as a booster dose for all adults.
Leer más »
 Moderna seeks FDA authorization for 4th dose of COVID shotWASHINGTON (AP) — Drugmaker Moderna asked the Food and Drug Administration on Thursday to authorize a fourth shot of its COVID-19 vaccine as a booster dose for all adults. The request is broader than rival pharmaceutical company Pfizer's request earlier this week for the regulator to approve a booster shot for all seniors.
Moderna seeks FDA authorization for 4th dose of COVID shotWASHINGTON (AP) — Drugmaker Moderna asked the Food and Drug Administration on Thursday to authorize a fourth shot of its COVID-19 vaccine as a booster dose for all adults. The request is broader than rival pharmaceutical company Pfizer's request earlier this week for the regulator to approve a booster shot for all seniors.
Leer más »
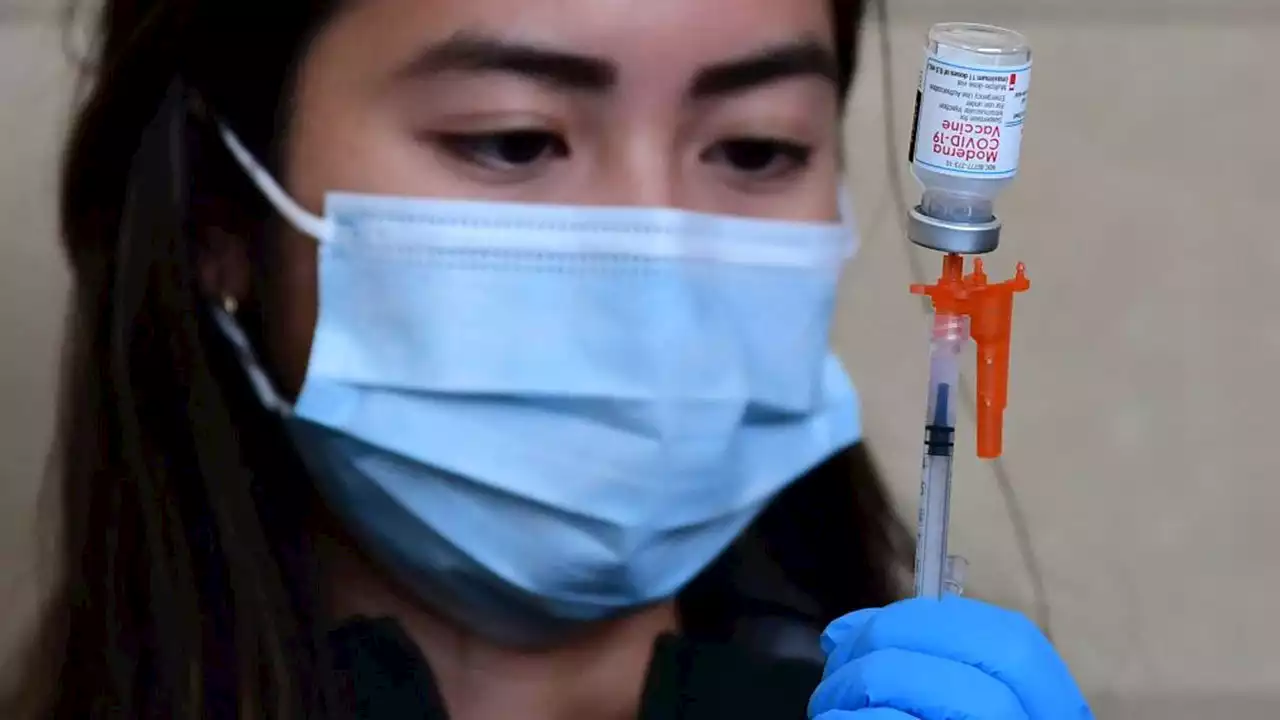 Moderna asks FDA to add 4th COVID-19 vaccine booster dose for all adults to EUADrugmaker Moderna has asked the Food and Drug Administration to authorize a fourth shot of its COVID-19 vaccine as a booster dose for all adults.
Moderna asks FDA to add 4th COVID-19 vaccine booster dose for all adults to EUADrugmaker Moderna has asked the Food and Drug Administration to authorize a fourth shot of its COVID-19 vaccine as a booster dose for all adults.
Leer más »
 New rapid PCR COVID test receives FDA emergency use authorizationA new rapid PCR COVID-19 test invented by Northwestern University faculty and Minute Molecular Diagnostics has received FDA emergency use authorization.
New rapid PCR COVID test receives FDA emergency use authorizationA new rapid PCR COVID-19 test invented by Northwestern University faculty and Minute Molecular Diagnostics has received FDA emergency use authorization.
Leer más »