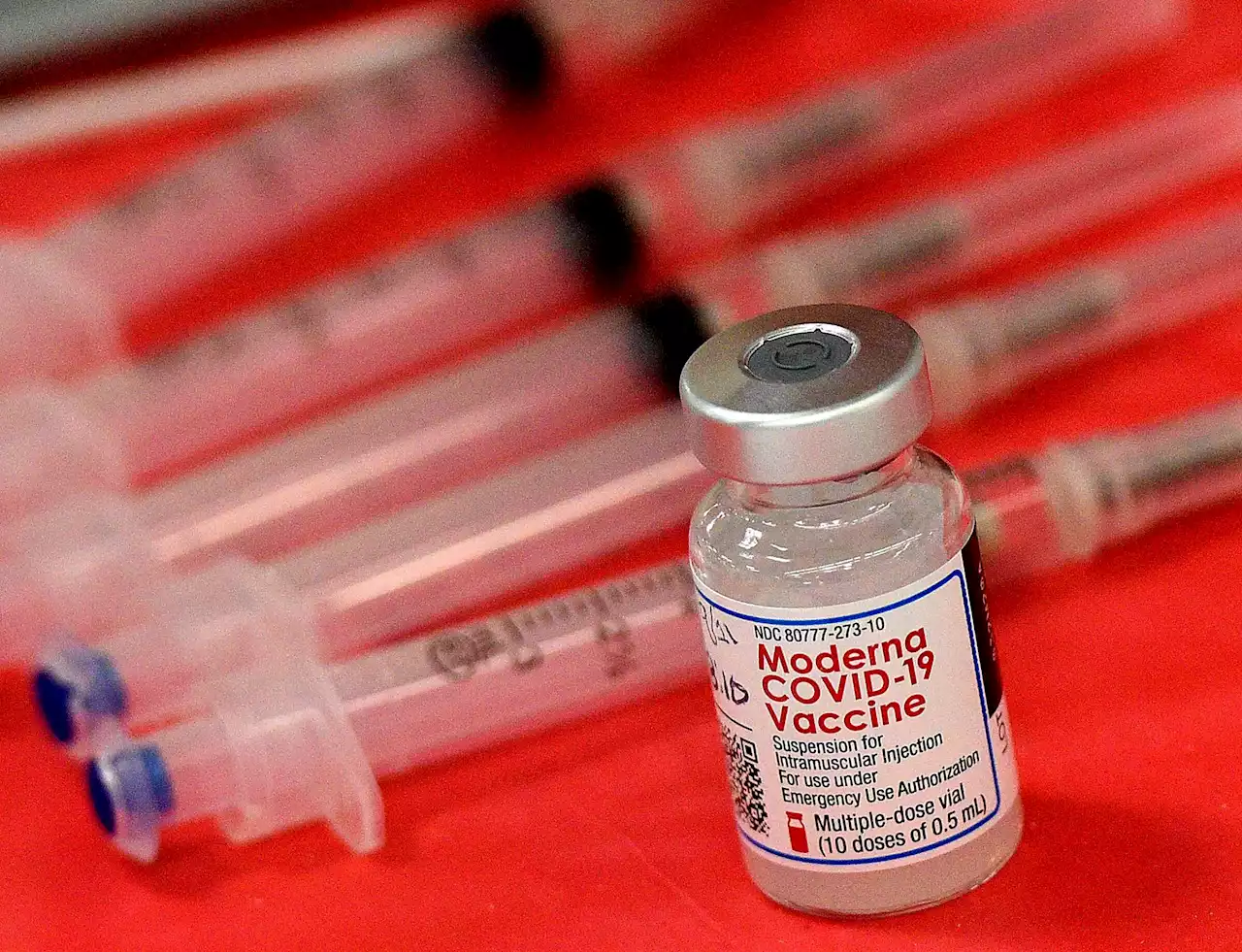
Moderna is asking U.S. regulators to open its COVID-19 vaccine to children younger than 6.
Moderna on Thursday asked U.S. regulators to authorize low doses of its COVID-19 vaccine for children younger than 6, a long-awaited move toward potentially opening shots for millions of tots by summer.
Now, only children ages 5 or older can be vaccinated in the U.S., using rival Pfizer’s vaccine, leaving 18 million younger tots unprotected. “It’s critically important that we have the proper evaluation so that parents will have trust in any vaccines that we authorize,” Marks told a Senate committee. The family continues to mask and take other precautions until it’s clear if the boys got real vaccine or dummy shots. If it turns out they weren’t protected in the Pfizer study and Moderna’s shots are cleared first, Dunphy-Daly said she’d seek them for her sons.
“This strain of COVID feels almost impossible to dodge,” Dana Walker, a mother of an 8-month-old, tearfully told a CDC meeting last week. “Cut red tape and allow parents to protect their kids.”In a study of kids ages 6 months through 5 years, two Moderna shots — each a quarter of the regular dose — triggered high levels of virus-fighting antibodies, the same amount proven to protect young adults, Burton said.
México Últimas Noticias, México Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 Moderna seeks COVID-19 vaccine authorization for children younger than age 5BREAKING: Moderna said it is seeking emergency use authorization for its COVID-19 vaccine for children as young as 6 months old.
Moderna seeks COVID-19 vaccine authorization for children younger than age 5BREAKING: Moderna said it is seeking emergency use authorization for its COVID-19 vaccine for children as young as 6 months old.
Leer más »
 Moderna seeks emergency authorization for COVID-19 vaccine in young childrenNEW: Moderna has submitted a request to the FDA for an emergency use authorization for its COVID-19 vaccine in children 6 months to under 6 years of age.
Moderna seeks emergency authorization for COVID-19 vaccine in young childrenNEW: Moderna has submitted a request to the FDA for an emergency use authorization for its COVID-19 vaccine in children 6 months to under 6 years of age.
Leer más »
 Moderna Asks FDA to Approve Its COVID-19 Shot for Kids Under 6Moderna on Thursday asked U.S. regulators to authorize low doses of its COVID-19 vaccine for children younger than 6, a long-awaited move toward potentially opening shots for millions of tots by summer.
Moderna Asks FDA to Approve Its COVID-19 Shot for Kids Under 6Moderna on Thursday asked U.S. regulators to authorize low doses of its COVID-19 vaccine for children younger than 6, a long-awaited move toward potentially opening shots for millions of tots by summer.
Leer más »
 Moderna Asks FDA to Clear Its Covid-19 Vaccine for Young ChildrenModerna asked the FDA to authorize its Covid-19 vaccine for children 6 months to 5 years. If regulators agree, vaccinations could begin by summer.
Moderna Asks FDA to Clear Its Covid-19 Vaccine for Young ChildrenModerna asked the FDA to authorize its Covid-19 vaccine for children 6 months to 5 years. If regulators agree, vaccinations could begin by summer.
Leer más »
 Moderna asks FDA to approve COVID-19 vaccine for kids under 6Moderna is asking U.S. regulators to approve its low-dose COVID-19 vaccine for children younger than 6, potentially opening shots for millions of tots by summer.
Moderna asks FDA to approve COVID-19 vaccine for kids under 6Moderna is asking U.S. regulators to approve its low-dose COVID-19 vaccine for children younger than 6, potentially opening shots for millions of tots by summer.
Leer más »
 Moderna asks FDA for authorization of its COVID-19 vaccine for children under 6The submission marks a hopeful development in the long journey for parents who are desperate to get their young kids vaccinated.
Moderna asks FDA for authorization of its COVID-19 vaccine for children under 6The submission marks a hopeful development in the long journey for parents who are desperate to get their young kids vaccinated.
Leer más »