The FDA is warning that parents should avoid using neck floats for infants with special needs or developmental delays.
. Some of these products are being marketed for infants as young as 2 weeks old, as well as for premature babies. But the FDA said the safety and effectiveness of the products for these children have not been proved.
The floats “have not been evaluated by the FDA and we are not aware of any demonstrated benefit with the use of neck floats for water therapy interventions,” the agency said in the June 28 statement. While injuries and deaths from neck floats are rare, the FDA said families and caregivers should be aware that these incidents can and do occur., the FDA Safety Information and Adverse Event Reporting Program. Health care personnel employed by the FDA are required to file new reports with the FDA.FDA: “Do Not Use Baby Neck Floats Due to the Risk of Death or Injury: FDA Safety Communication.”
México Últimas Noticias, México Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 FDA Warns Against Baby Neck Floats After A Reported DeathThe organization urged caregivers to stop using inflatable neck floats, especially with babies who have developmental delays or special needs.
FDA Warns Against Baby Neck Floats After A Reported DeathThe organization urged caregivers to stop using inflatable neck floats, especially with babies who have developmental delays or special needs.
Leer más »
 FDA temporarily suspends order banning Juul cigarettesThe Food and Drug Administration has issued a stay of its recent order for vaping company Juul to pull its electronic cigarettes from the market. The FDA says the stay is temporary while it conducts further review.
FDA temporarily suspends order banning Juul cigarettesThe Food and Drug Administration has issued a stay of its recent order for vaping company Juul to pull its electronic cigarettes from the market. The FDA says the stay is temporary while it conducts further review.
Leer más »
 'Unique' Issues Cause FDA To Temporarily Suspend Ban On Juul CigarettesThe FDA issued the initial order banning Juul sales on June 23. A day later, a federal appeals court temporarily blocked the government ban.
'Unique' Issues Cause FDA To Temporarily Suspend Ban On Juul CigarettesThe FDA issued the initial order banning Juul sales on June 23. A day later, a federal appeals court temporarily blocked the government ban.
Leer más »
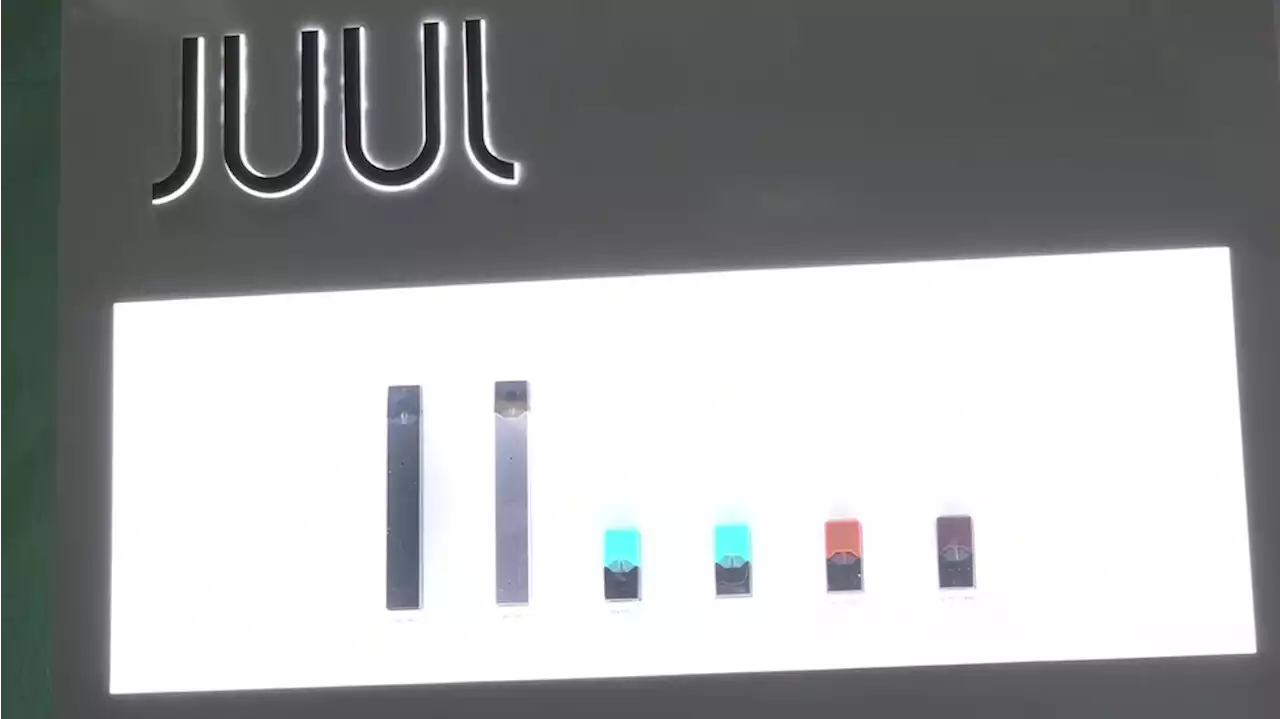 FDA temporarily suspends order banning Juul cigarettesThe Food and Drug Administration said on Twitter that the stay temporarily suspends the marketing denial order while it conducts further review, but does not rescind it.
FDA temporarily suspends order banning Juul cigarettesThe Food and Drug Administration said on Twitter that the stay temporarily suspends the marketing denial order while it conducts further review, but does not rescind it.
Leer más »
 FDA decision on Eisai, Biogen Alzheimer's drug due in JanuaryThe U.S. Food and Drug Administration will expedite its review of Eisai Co Ltd's and Biogen Inc's experimental Alzheimer's drug lecanemab, with a decision due by Jan 6, 2023, the companies said on Tuesday.
FDA decision on Eisai, Biogen Alzheimer's drug due in JanuaryThe U.S. Food and Drug Administration will expedite its review of Eisai Co Ltd's and Biogen Inc's experimental Alzheimer's drug lecanemab, with a decision due by Jan 6, 2023, the companies said on Tuesday.
Leer más »
 Juul Can Keep Selling Its Products in U.S. While It Appeals Ban, FDA SaysThe FDA says it will allow Juul to stay on the U.S. market while the e-cigarette maker appeals the agency’s ban
Juul Can Keep Selling Its Products in U.S. While It Appeals Ban, FDA SaysThe FDA says it will allow Juul to stay on the U.S. market while the e-cigarette maker appeals the agency’s ban
Leer más »
