Pfizer and German partner BioNTech have submitted their new COVID-19 booster – that targets the omicron subvariant BA.5 – to the FDA for emergency use authorization.
The CDC is responding to criticism over its handling of the COVID-19 pandemic, monkeypox and other public health threats.Pfizer and German partner BioNTech have submitted their new COVID-19 booster – that targets the omicron subvariant BA.
The company has tested the BA.5-specific vaccine only on mice, so far, and is relying on data from both the BA.1 human trials and the BA.5 mice trials for their submission for authorization. “To rely only on mouse data would be unprecedented in my knowledge and would certainly raise eyebrows,” said John Moore, a vaccine and virology expert at Weill Cornell Medicine in New York. “It doesn’t mimic the human situation,” where many people were vaccinated more than a year ago and have since been boosted.
But Moore isn't convinced the data will show the BA.5 booster working significantly better than the BA.1 booster, or even the original booster. The public expects the new booster to prevent more infections, he said, but it will probably be only marginally more protective.
México Últimas Noticias, México Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 Pfizer-BioNTech Submits the First Omicron COVID-19 Booster for FDA AuthorizationPfizer-BioNTech Submit the First Omicron COVID-19 Booster for FDA Authorization
Pfizer-BioNTech Submits the First Omicron COVID-19 Booster for FDA AuthorizationPfizer-BioNTech Submit the First Omicron COVID-19 Booster for FDA Authorization
Leer más »
 Pfizer, BioNTech Seek FDA Authorization for Updated Covid-19 VaccineThe updated shot, which targets the latest Omicron subvariants as well as the original virus, would be used in a fall booster campaign.
Pfizer, BioNTech Seek FDA Authorization for Updated Covid-19 VaccineThe updated shot, which targets the latest Omicron subvariants as well as the original virus, would be used in a fall booster campaign.
Leer más »
 Pfizer asks FDA to authorize updated COVID vaccine adding protection from Omicron variantsThe FDA ordered vaccine makers to tweak their shots to target BA.4 and BA.5. Pfizer and its partner BioNTech aim to offer updated boosters to people 12 and older, and shots could begin within weeks if the FDA quickly clears the modified vaccine.
Pfizer asks FDA to authorize updated COVID vaccine adding protection from Omicron variantsThe FDA ordered vaccine makers to tweak their shots to target BA.4 and BA.5. Pfizer and its partner BioNTech aim to offer updated boosters to people 12 and older, and shots could begin within weeks if the FDA quickly clears the modified vaccine.
Leer más »
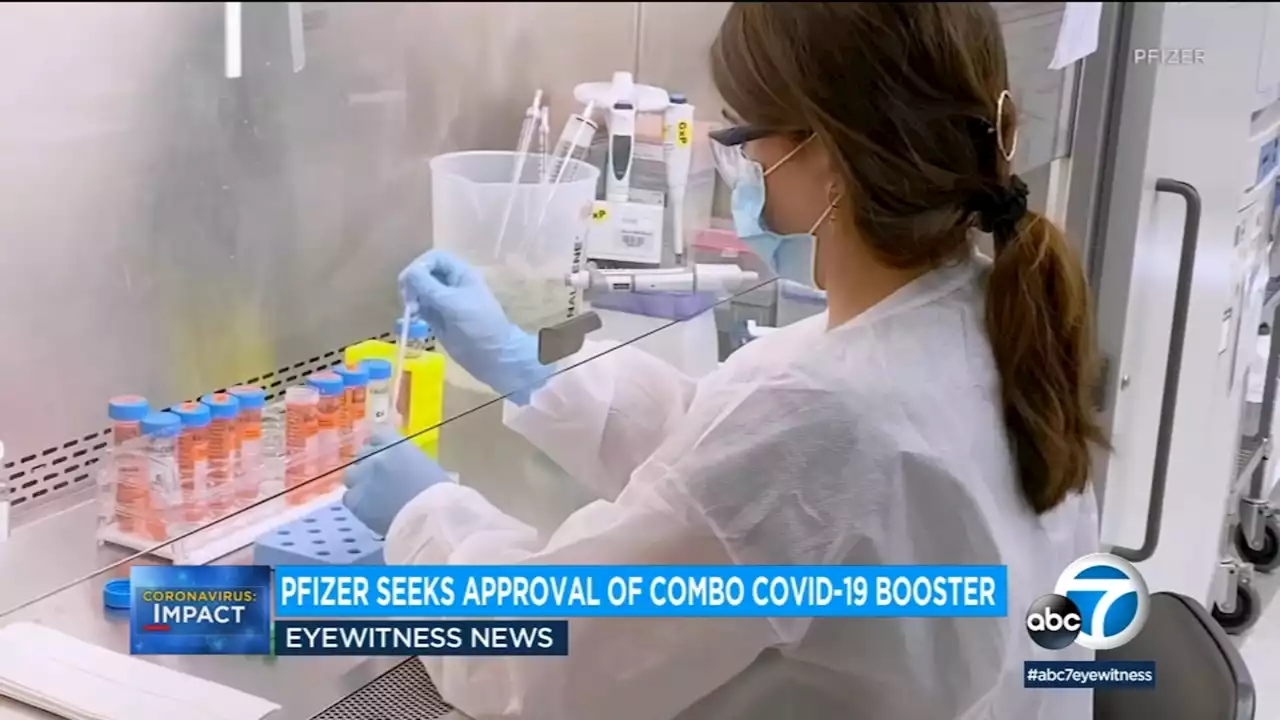 Pfizer asks FDA to authorize updated COVID vaccine adding protection from Omicron variantsThe FDA ordered vaccine makers to tweak their shots to target BA.4 and BA.5. Pfizer and its partner BioNTech aim to offer updated boosters to people 12 and older, and shots could begin within weeks if the FDA quickly clears the modified vaccine.
Pfizer asks FDA to authorize updated COVID vaccine adding protection from Omicron variantsThe FDA ordered vaccine makers to tweak their shots to target BA.4 and BA.5. Pfizer and its partner BioNTech aim to offer updated boosters to people 12 and older, and shots could begin within weeks if the FDA quickly clears the modified vaccine.
Leer más »
 Pfizer seeks OK of updated COVID vaccine booster for fallPfizer has asked U.S. regulators to authorize its combination COVID-19 vaccine that adds protection against the newest omicron relatives.
Pfizer seeks OK of updated COVID vaccine booster for fallPfizer has asked U.S. regulators to authorize its combination COVID-19 vaccine that adds protection against the newest omicron relatives.
Leer más »