Pfizer Inc Chief Executive Albert Bourla said on Saturday that an annual COVID-19 vaccine would be preferable to more frequent booster shots in fighting the coronavirus pandemic.
Chief Executive Albert Bourla said on Saturday that an annual COVID-19 vaccine would be preferable to more frequent booster shots in fighting the coronavirus pandemic.COVID-19 vaccine has shown to be effective against severe disease and death caused by the heavily-mutated Omicron variant but less effective in preventing transmission.
"This will not be a good scenario. What I'm hoping that we will have a vaccine that you will have to do once a year," Bourla said.Pfizer CEO Albert Bourla talks during a press conference with European Commission President Ursula von der Leyen after a visit to oversee the production of the Pfizer-BioNtech COVID-19 vaccine at the factory of U.S. pharmaceutical company Pfizer in Puurs, Belgium April 23, 2021.
Bourla has said Pfizer could be ready to file for approval for a redesigned vaccine to fight Omicron, and mass produce it, as soon as March.
México Últimas Noticias, México Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 Annual COVID vaccine preferable to boosters, says Pfizer CEO'What I'm hoping (is) that we will have a vaccine that you will have to do once a year,' Bourla said.
Annual COVID vaccine preferable to boosters, says Pfizer CEO'What I'm hoping (is) that we will have a vaccine that you will have to do once a year,' Bourla said.
Leer más »
 Pfizer COVID-19 Vaccine for Kids Under 5 Could be Authorized Soon: OfficialsPediatricians say the omicron surge has been especially hard on children and there may be relief soon for those under 5.
Pfizer COVID-19 Vaccine for Kids Under 5 Could be Authorized Soon: OfficialsPediatricians say the omicron surge has been especially hard on children and there may be relief soon for those under 5.
Leer más »
 Third Dose of Pfizer, Moderna Covid-19 Vaccines Offers Strong Protection Against OmicronCDC analysis shows that boosters are important in maximizing protection against Omicron and Delta variants.
Third Dose of Pfizer, Moderna Covid-19 Vaccines Offers Strong Protection Against OmicronCDC analysis shows that boosters are important in maximizing protection against Omicron and Delta variants.
Leer más »
 Bay Area job market soars to big gains in December, defies COVID surgeThe Bay Area added jobs at a brisk pace in December, ending 2021 on a bright note that defied the fresh economic uncertainties unleashed by the omicron variant of the coronavirus.
Bay Area job market soars to big gains in December, defies COVID surgeThe Bay Area added jobs at a brisk pace in December, ending 2021 on a bright note that defied the fresh economic uncertainties unleashed by the omicron variant of the coronavirus.
Leer más »
 FDA acts to expand use of treatment for COVID-19 patients with mild-to-moderate diseasePreviously, the use of Veklury, a Gilead Sciences Inc. drug, was limited to those requiring hospitalization.
FDA acts to expand use of treatment for COVID-19 patients with mild-to-moderate diseasePreviously, the use of Veklury, a Gilead Sciences Inc. drug, was limited to those requiring hospitalization.
Leer más »
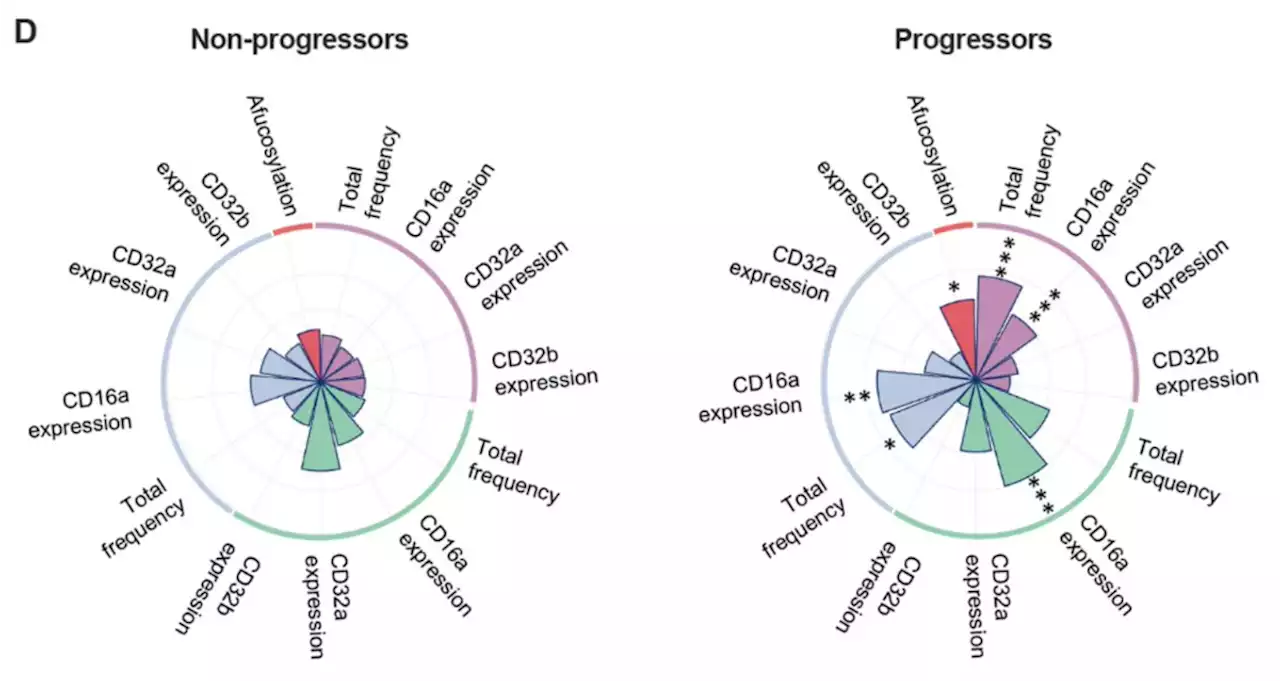 Early non-neutralizing, afucosylated antibody responses are associated with COVID-19 severityUnlike antibodies elicited by mRNA vaccines against COVID19, some non-neutralizing, afucosylated IgG antibodies specific to SARSCoV2 may be associated with progression to more severe disease, according to new research in ScienceTM.
Early non-neutralizing, afucosylated antibody responses are associated with COVID-19 severityUnlike antibodies elicited by mRNA vaccines against COVID19, some non-neutralizing, afucosylated IgG antibodies specific to SARSCoV2 may be associated with progression to more severe disease, according to new research in ScienceTM.
Leer más »
