Anyone 50 and older could soon be eligible for a second booster dose of the Moderna or Pfizer-BioNtech COVID-19 vaccine. The plan comes as evidence increases that protection from three shots is fading and a fourth shot would help boost immunity back up.
The plan comes as evidence increases that protection from three shots is fading and a fourth shot would help boost immunity back up. And asversion of the omicron variant, continues to spread in the U.S., concern is mounting it could fuel another surge.Dr. Eric Topol,“Without protection against the omicron variant, particularly now we’re confronting BA.2, there’s a very high risk of hospitalization and death,” he says.
“From a scientific perspective, we still don’t have definitive evidence that giving a second booster dose is the right way to go in older people,” saysshows an additional booster dose does reduce the risk of severe disease, hospitalization and death for people over the age of 60. But she points out it’s unclear how long that extra protection actually lasts.an infectious disease researcher at Emory University told NPR.
But other infectious disease specialists say the administration should be focusing on getting people their primary doses and first boosters.
México Últimas Noticias, México Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 The FDA is expected to authorize 2nd boosters for people 50 and upPeople aged 50 and over could soon be eligible for a second Pfizer-BioNTech or Moderna COVID vaccine booster. The administration wants to offer the shots as immunity from the first booster is waning.
The FDA is expected to authorize 2nd boosters for people 50 and upPeople aged 50 and over could soon be eligible for a second Pfizer-BioNTech or Moderna COVID vaccine booster. The administration wants to offer the shots as immunity from the first booster is waning.
Leer más »
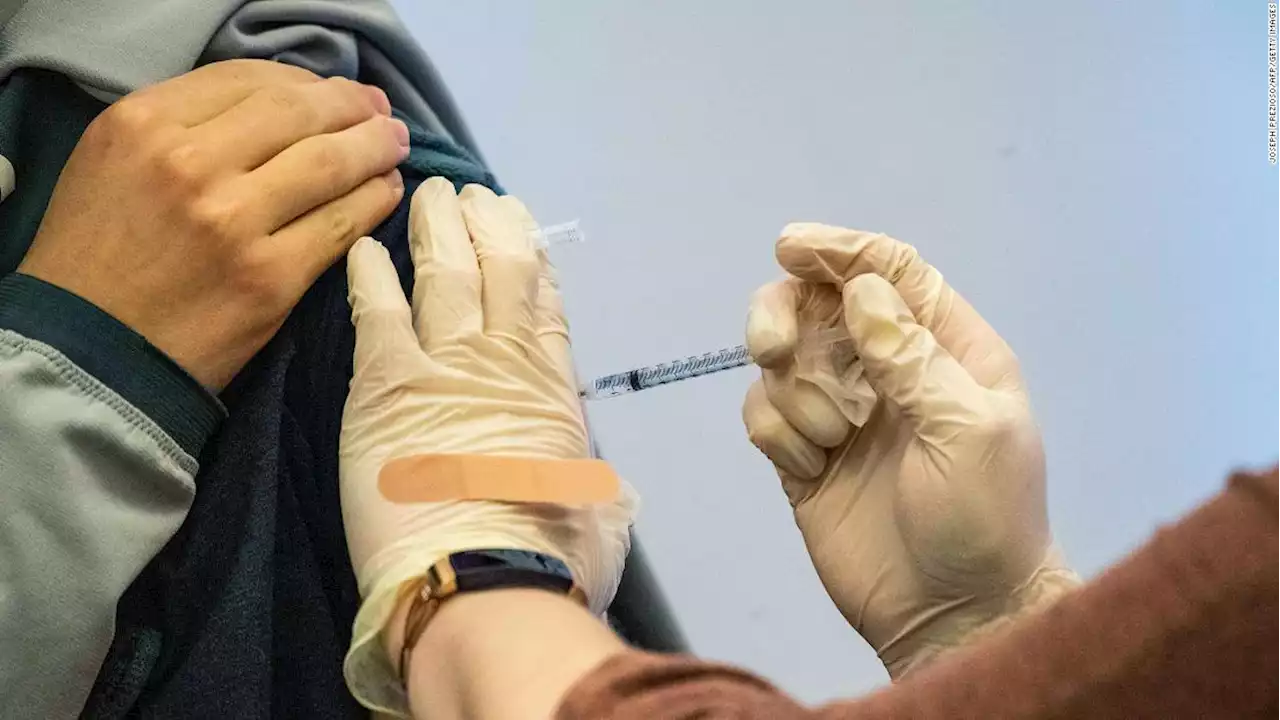 FDA expected to OK additional Covid-19 booster shots for adults over 50 next weekTwo sources familiar with the government's plans said the US Food and Drug Administration is planning authorize a fourth dose of the Pfizer and Moderna mRNA vaccines for adults over age 50 next week.
FDA expected to OK additional Covid-19 booster shots for adults over 50 next weekTwo sources familiar with the government's plans said the US Food and Drug Administration is planning authorize a fourth dose of the Pfizer and Moderna mRNA vaccines for adults over age 50 next week.
Leer más »
 ABC News: FDA could authorize second booster shots for Americans over 50 this weekTwo officials familiar with the matter tell ABC News the FDA could authorize second booster shots for Americans over 50 years old as soon as Tuesday, but the details are still under discussion.
ABC News: FDA could authorize second booster shots for Americans over 50 this weekTwo officials familiar with the matter tell ABC News the FDA could authorize second booster shots for Americans over 50 years old as soon as Tuesday, but the details are still under discussion.
Leer más »
 FDA expected to authorize second coronavirus booster for 50 and olderThe FDA is poised to authorize a second coronavirus vaccine booster for anyone 50 and older. It would provide an extra layer of protection amid concerns Europe’s rise in infections from an omicron subvariant could hit the United States.
FDA expected to authorize second coronavirus booster for 50 and olderThe FDA is poised to authorize a second coronavirus vaccine booster for anyone 50 and older. It would provide an extra layer of protection amid concerns Europe’s rise in infections from an omicron subvariant could hit the United States.
Leer más »
 Americans over 50 could soon have option of getting 2nd COVID booster shotThe Biden administration is expected to give Americans over 50 the option to get a second COVID-19 booster shot, according to reports.
Americans over 50 could soon have option of getting 2nd COVID booster shotThe Biden administration is expected to give Americans over 50 the option to get a second COVID-19 booster shot, according to reports.
Leer más »
